Abstract
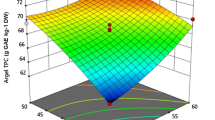
Ultrasonication technology was utilized for extraction of effective components from Nepeta (Nepeta binaludensis Jamzad). Response surface methodology was employed in order for investigation the effects of independent process variables (ultrasonic extraction time (UET, min): 5, 10 and 15 min, ultrasound amplitude (UA, %): 20, 60 and 100%, ultrasonic extraction temperature (UEC, °C): 25, 35 and 45 °C and ultrasonic duty cycle (UDC, %): 20, 60 and 100%) on the yield (Y), total phenolic compounds (TPC), half maximal of radical (1,1-diphenyl-2-picryl hydrazyl, DPPH) scavenging activity (IC50) and ferric reducing-antioxidant power (FRAP) of ethanolic Nepeta extract. According to Derringer’s desired function approach, the optimal conditions based on both individual and combinations of all process variables were UET 15 min, UA 60%, UEC 25 °C and UDC 60%. At this optimum condition, the Y, TPC, IC50 and FRAP of the ultrasound-assisted extract (USx) were found to 10.93%, 402.63 mg GA/kg, 0.33 mg/mL and 2838.34 µmol Fe2+/kg, respectively which were well matched with the predicted values. The evaluation of antioxidant activity (IC50 and FRAP) indicates that the phenolic compounds from Nepeta possess significant antioxidant activity. Also, HPLC analysis revealed that at optimal condition of UAE, the quantity of caffeic acid (85.71%), rutin (3550%) and rosemarinic acid (468.62%) in extract increased in comparison with conventional method (solvent extraction).




Similar content being viewed by others
Abbreviations
- UAE:
-
Ultrasound assisted extraction
- FCD:
-
Face-centered experimental design
- RSM:
-
Response surface methodology
- Y:
-
Yield
- TPC:
-
Total phenolic compounds
- DPPH:
-
Radical scavenging activity of DPPH
- FRAP:
-
Ferric reducing-antioxidant power
- IC50 :
-
50% of Radical-scavenging Activity
- UA:
-
Ultrasonic amplitude
- UET:
-
Ultrasonic exposure time
- UEC:
-
Ultrasonic extraction temperature
- UDC:
-
Ultrasonic duty cycle USx
- SOx:
-
Ultrasound-assisted extract solvent extract
References
F. Shahidi, J. Yeo, Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int. J. Mol. Sci. 19, 1–16 (2018)
S.M.F. Bessada, J.C.M. Barreira, L. Barros, I.C.F.R. Ferreirab, M. Beatriz, P. Oliveira, Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f: an underexploited and highly disseminated species. Ind. Crops Prod. 89, 45–51 (2016)
A. Shakeri, F. Khakdan, V. Soheili, A. Sahebkar, G. Rassam, J. Asilia, Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crops Prod. 58, 315–321 (2014)
F. Nadjafi, A. Koocheki, P. Rezvani Moghaddam, B. Honermeier, First experiments on cultivation of Nepeta binaludensis Jamzad—an example of domestication of a highly endangered medicinal plant of Iran. Z Arznei Gewurzpflanzen, 17, (2012)
S. Pimentel-Moral, I. Borrás-Linares, J. Lozano-Sánchez, D. Arráez-Román, A. Martínez-Férez, A. Segura-Carretero, Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 156, 313–322 (2018)
Y. Poodi, M. Bimakr, A. Ganjloo, S. Zarringhalami, Intensification of bioactive compounds extraction from Feijoa (Feijoa sellowiana Berg.) leaves using ultrasonic waves. Food Bioprod. Process. 108, 37–50 (2018)
I. Bozinou, G. Karageorgou, V.G. Batra, S.I. Dourtoglou, Lalas. Pulsed electric field extraction and antioxidant activity determination of moringa oleifera dry leaves: a comparative study with other extraction techniques, beverages 5(1), 1–13 (2019)
T. Hatami, J. Cezar Flores Johner, M.A.A. Meireles, Extraction and fractionation of fennel using supercritical fluid extraction assisted by cold pressing. Ind. Crops Prod. 123, 661–666 (2018)
F. Feng, Z. Luo, B. Tao, C.H. Chen, Ultrasonic-assisted extraction and purification of phenolic compounds from sugarcane (Saccharum officinarum L.) rinds. LWT Food Sci. Technol. 60, 970–976 (2015)
B. He, L.L. Zhang, X.Y. Yue, J. Liang, J. Jiang, X.L. Gao, P.X. Yue, Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 204, 70–76 (2016)
R.H. Myers, D.C. Montgomery, C.M. Anderson-Cook, Response surface methodology: process and product optimization using designed experiments (Wiley, New York, 2016)
T. Belwal, S.M. Ezzat, L. Rastrelli, I.D. Bhatt, M. Daglia, A. Baldi, H.P. Devkota, I.E. Orhan, J.K. Patra, G. Das, C. Anandharamakrishnan, L. Gomez-Gomez, S.F. Nabavi, S.M. Nabavi, A.G. Atanasov, A critical analysis of extraction techniques used for botanicals: trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 100, 82–102 (2018)
M. Irakli, P. Chatzopoulou, L. Ekateriniadou, Optimization of ultrasound-assisted extraction of phenolic compounds: leuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 124, 382–388 (2018)
N.R. Mihailović, V.B. Mihailović, A.R. Ćirić, N.Z. Srećković, M.R. Cvijović, L.G. Joksov, Analysis of wild raspberries optimization of the ultrasonic-assisted extraction of phenolics and a new insight in phenolics bio accessibility. Plant Foods Hum. Nutr. 74, 399–404 (2019)
N. Mohammadpour, S.A. Emami, J. Asili, Identification of volatile oil components of Nepeta binaludensis Jamzad by GC-MS and 13C-NMR methods and evaluation of its antimicrobial activity. J. Essent. Oil Bearing Plants 16(1), 102–107 (2013)
Z. Tayarani-Najaran, M. Akaberi, M. Vatani, S.A. Emami, Evaluation of antioxidant and anti-melanogenic activities of different extracts from aerial parts of Nepeta binaludensis Jamzad in murine melanoma B16F10 cells. Iran. J. Basic Med. Sci. 19(6), 662–669 (2016)
P. Sharayei, E. Azarpazhooh, S.H. Zomorodi, H.S. Ramaswamy, Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT Food Sci. Technol. 101, 342–352 (2019)
F.C. Eze, L. Ngonadi, Alphabetic optimality criteria for 2 K central composite design. Acad. J. Appl. Math. Sci. 4(9), 107–118 (2018)
E. Azarpazhooh, H.S. Ramaswamy, Modeling and optimization of microwave osmotic dehydration of apple cylinders under continuous-flow spray mode processing conditions. Food Bioprocess Technol. 5(5), 1486–1501 (2012)
V.L. Singleton, R. Orthofer, R.M. Lamuela-Raventos, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299, 152–178 (1999)
A. Siger, M. Nogala-Kalucka, E. Lampart-Szczapa, The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 15, 137–149 (2007)
I.F.F. Benzie, J.J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239, 70–76 (1996)
W. Zheng, S.Y. Wang, Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 49(11), 5165–5170 (2001)
Q. Beg, V. Sahai, R. Gupta, Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process. Biochem. 39, 203–209 (2003)
A. Tomšik, B. Pavlic, J. Vladic, M. Ramic, J. Brindza, S. Vidovic, Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 29, 502–511 (2016)
M. Rouhani, Modeling and optimization of ultrasound-assisted green extraction and rapid HPTLC analysis of stevioside from Stevia Rebaudiana. Ind. Crops Prod. 132, 226–235 (2019)
J. Wang, B. Sun, Y. Cao, Y. Tian, X. Li, Optimization of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 106, 804–810 (2008)
Y. Jina, Z. Liua, D. Liuc, G. Shia, D. Liud, Y. Yange, H. Guf, L. Yanga, Z. Zhoua, Natural antioxidant of rosemary extract used as an additive in the ultrasound-assisted extraction of anthocyanins from lingonberry (Vaccinium vitis- idaea L.) pomace. Ind. Crops Prod. 138, 111425 (2019)
F. Dahmoune, H. Remini, S. Dairi, O. Aoun, K. Moussi, N. Bouaoudia-Madi, N. Adjeroud, N. Kadri, K. Lefsih, L. Boughani, L. Mouni, Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. Ind. Crops Prod. 77, 251–261 (2015)
G. Sharmila, V.S. Nikitha, S. Ilaiyarasia, K. Dhivya, V. Rajasekar, N.M. Kumar, K. Muthukumaran, C. Muthukumaran, Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 84, 13–21 (2016)
Y. Chavan, R.S. Singhal, Ultrasound-assisted extraction (UAE) of bioactives from arecanut and optimization study using response surface methodology. Innov. Food Sci. Emerg. Technol. 17, 106–113 (2013)
M. Kozarski, A. Klaus, M. Niksic, D. Jakovljevic, J.P.F.G. Helsper, L.J.L.D. Van Griensven, Antioxidative and immune modulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Gano dermalucidum and Phellinus linteus. Food Chem. 129, 1667–1675 (2011)
A. Moure, J.M. Cruz, D. Franco, J.M. Dominguez, J. Sineiro, H. Dominguez, M.J. Nunez, J.C. Parajo, Natural antioxidants from residual sources. Food Chem. 72, 145–171 (2001)
A. Gokbulut, Validated RP- HPLC method for quantification of phenolic compounds in methanol extracts of aerial parts and roots of Thymus sipyleus and evaluation of antioxidant potential. Trop. J. Pharm. Res. 14(10), 1871–1877 (2015)
M. Krizman, D. Baričevic, M. Prosek, Determination of phenolic compounds in fennel by HPLC and HPLC–MS using a monolithic reversed- phase column. J. Pharm. Biomed. Anal. 43(2), 481–485 (2007)
Z. Guan, S. Li, Z. Lin, R. Yang, Y. Zhao, J. Liu, S. Yang, A. Chen, Identification and quantitation of phenolic compounds from the seed and pomace of Perilla frutescens using HPLC/PDA and HPLC–ESI/QTOF/MS/MS. Phytochem. Anal. 25(6), 508–513 (2014)
S. Han, S. Yang, Z. Cai, D. Pan, Z. Li, Z. Huang, P. Zhang, H. Zhu, L. Lei, W. Wang, Anti-Warburg effect of rosemarinic acid via miR- 155 in gastric cancer cells. Drug Des. Dev. Ther. 9, 2695 (2015)
A. Mazumder, N. Neamati, S. Sunder, J. Schulz, H. Pertz, E. Eich, Y. Pommier, Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J. Med. Chem. 40(19), 3057–3063 (1997)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Afsaneh Azimi Mahalleh, Parvin Sharayei, and Elham Azarpazhooh declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azimi Mahalleh, A., Sharayei, P. & Azarpazhooh, E. Optimization of ultrasonic-assisted extraction of bioactive compounds from Nepeta (Nepeta binaludensis Jamzad). Food Measure 14, 668–678 (2020). https://doi.org/10.1007/s11694-019-00314-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00314-1