Abstract
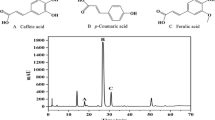
Extraction of ferulic acid from sugar beet pulp was carried out using three extraction solvents, sodium hydroxide (0.5, 1, 2 M), methanol and their mixture (alkaline methanolic solvent). The Ferulic acid extracted by each solvent was identified and quantified by HPLC method and the effects of solvent type, concentration and reaction time on ferulic acid solubilisation were assessed. There were differences in the contents of products extracted in the experiment conditions. The minimum amount of ferulic acid was obtained from methanolic extract while the highest concentrations (957.4 mg/L ferulic acid) were obtained employing the highest NaOH concentration (2 M), and reaction time (12 h), so phenolic compounds are better released with alkaline hydrolysis than in methanol conditions. Finally a simple procedure for the purification of ferulic acid from the alkaline extracts is presented and evaluated by FT-IR spectrum.


Similar content being viewed by others
References
C.D. Stalikas, J. Sep. Sci. 30, 3268–3295 (2007). doi:10.1002/jssc.200700261
D.R. Rose, G.E. George, S.X. Liu. J. Sci. Food. Agr. 90, 519–524 (2010)
H.Z. Zhao, M.H. Moghadasian, Food Chem. 109, 691–702 (2008). doi:10.1016/j.foodchem.2008.02.039
J.M. Cruz, J.M. Dominguez, H. Domınguez, J.C. Parajo, J. Agr. Food. Chem 49, 2459–2464 (2001). doi:10.1021/jf001237h
J. Gonzalez, J.M. Cru, H. Dominguez, J.C. Parajo, Food Chem. 84, 503–510 (2004). doi:10.1016/S0308-8146(03)00208-5
C.B. Faulds, B. Bartolome, G. Williamson, Ind. Crops Prod. 6, 367–374 (1997)
P. Jankovska, J. Copikova, A. Sinitsya, Czech. J. Food. Sci. 19, 4 (2001)
B.A. Acosta-Estrada, J.A. Gutierrez-Uribe, S.O. Serna-Saldivar, Food Chem 152, 46–55 (2014). doi:10.1016/j.foodchem.2013.11.093
D. Couteau, P. Mathaly, Ind. Crops Prod. 6, 237–252 (1997)
G.H. Lu, K. Chan, K. Leung, C.L. Chan, Z.H. Zhao, Z.H. Jiang, J. Chromatogr. A 1068, 209–219 (2005)
N.B. Dar, S. Sharma, Am. J. Food Tech. 6, 12 (2011)
M. Bunzel, J. Ralph, C. Funk, H. Steinhart, Eur. Food Res. Technol. 217, 128–133 (2003). doi:10.1007/s00217-003-0709-0
L. Zheng, Pu Zheng, Z. Sun, Y. Bai, J. Wang, X. Guo, Bioresour. Technol. 98, 1115–1119 (2007). doi:10.1016/j.biortech.2006.03.028
B. Max, A. M. Torrado, A. B. Moldes, A. Converti, J.M. Domingueza, Biochem. Eng. J., 43 (2009). doi:10.1016/j.indcrop.2006.11.001
H. Barberousse, O. Olivie Roiseux, C. Robert, M. Paquot, C. Deroanne, C. Blecker, J. Sci. Food Agric. 88, 1494–1511 (2008). doi:10.1002/jsfa.3242
A.U. Buranov, G. Mazza, Food Chem. 115, 1542–1548 (2009). doi:10.1016/j.foodchem.2009.01.059
L. Kunst, A.L. Samuels, Prog. Lipid Res. 42, 51–80 (2003)
D.S. Oufnac, Z. Xu, T. Sun, C. Sabliov, W. Prinyawiwatkul, J.S. Godber, Cereal Chem. 84, 2 (2007)
P. Torre, B. Aliakbarian, B. Rivas, J.M. Dominguez, A. Attilio Converti, Biochem. Eng. J. 40, 500–506 (2008). doi:10.1016/j.bej.2008.02.005
M.S. Noor Hasyierah, M.D. Mohamed Zulkali, A. Dachyar, A.M. Syarhabil, K.I. Ku Syahidah, Ind. Crops. Prod. 34, 1635–1640 (2011). doi:10.1016/j.indcrop.2011.06.010
J.L. Bauer, B. Harbaum-Piayda, K. Schwarz, LWT- Food Sci. Technol. 47, 246–254 (2012). doi:10.1016/j.lwt.2012.01.012
Y. Yongyue Sun, W. Wang, J. Chin. Inst. Chem. Eng, 39, 653–656 (2008). doi:10.1016/j.jcice.2008.05.012
K.W. Waldron, N. Annie, L.P. Mary, J.P Adrian, J. Sci. Food Agric. 74, 221–228 (1997)
M.S. Robert, X.W. Francis, Text Book of Spectrometric Identification of Organic Compounds (Wiley, New York, 1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aarabi, A., Mizani, M., Honarvar, M. et al. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. Food Measure 10, 42–47 (2016). https://doi.org/10.1007/s11694-015-9274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-015-9274-z