Abstract
Phenotypic change plays diverse roles in species’ colonization, but most invasion studies target single species. To compare ecomorphological changes among co-invading species with overlapping niches, we examined three lizards on the island of O‘ahu (Anolis carolinensis, A. sagrei, Phelsuma laticauda). Using specimens from three decades of unfolding invasions obtained through museum collections and contemporary field work, we quantified shifts in three traits: snout vent length (SVL), forelimb-, and hindlimb-length (limb lengths relative to SVL). We hypothesized that competition among these three species has led to ecological shifts that will be detectable through morphological change. Overall, we found that unique patterns of phenotypic change were both species-specific and sex-specific within species: (1) male A. sagrei, female A. carolinensis, and male P. laticauda increased in SVL and (2) relative hindlimb length increased in female A. carolinensis since the 1980s. The observed changes involve traits that may be consequential to invasion dynamics. This study illustrates how museum- and field-based research can be integrated to document nuanced temporal patterns in the phenotypes of co-invading species that share similar niches in native ranges, raising questions about the underlying process(es) driving species- and sex-specific change in co-invaded systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid shifts in behavior or morphology have been observed in many invasive species and may represent adaptive exploitation of novel habitats (e.g., Avilés-Rodríguez & Kolbe, 2019). Most prior research in this area focuses on elucidating the patterns and processes of post-invasive change within a single taxon, and/or how focal taxa impact native ecosystems (Brown et al., 2013; Llewelyn et al., 2011; Phillips et al., 2006, 2010). However, when potential colonizers have overlapping niches, their impacts on novel ecosystems are dependent upon interactions among the co-invading species (Grosholz, 2005; Jackson, 2015; Mothes et al., 2019; Pringle et al., 2019; Stroud et al., 2020). Therefore, identifying patterns and drivers of change within co-invaded systems can help illuminate features of invasion biology, since accelerating rates of species’ dispersal present growing opportunities for divergent species to establish in sympatry outside their native ranges (Mothes et al., 2019; Stroud et al., 2020).
Among the classic model systems for the study of rapid evolution (particularly in the context of invasion) are Caribbean anole lizards (Jackman et al., 1999; Losos, 2009). Decades of research suggest that anole morphology and behavior predictably evolve via local adaptation and niche partitioning among species (Losos, 2009; Losos et al., 1997; Yuan et al., 2020). Morphological changes are also observable within Anolis species over very short time intervals (Donihue et al., 2018, 2020), with both selection and phenotypic plasticity known as driving forces subjacent to phenotypic change in Anolis (Kolbe & Losos, 2005; Losos et al., 2000; Winchell et al., 2023). For example, vegetation structure and predators drove somewhat predictable evolution over just a few years’ time in A. sagrei, with founder effects limiting variability (Kolbe et al., 2012). Rapid morphological change has also been observed in Caribbean anoles colonizing urban niches (Winchell et al., 2016, 2023), suggesting that phenotypic change may play a role in exploiting novel habitats. Therefore, Anolis lizards are an excellent model to test rapid evolution following ecological change across various evolutionary pathways.
Anoles are capable of rapid phenotypic change with clear and diverse links to fitness, sometimes driven by heterospecifics with overlapping niches. When introduced A. sagrei in the southern US encountered allopatric A. carolinensis, A. carolinensis shifted to higher perches and increased toepad size within 20 generations (Stuart et al., 2014). Introduced A. carolinensis have similarly undergone rapid morphological change in the Pacific Ogasawara Archipelago where hindlimb length increased relative to native Florida populations (Tamate et al., 2017). These changes are ecologically significant given the well-described relationships between focal (ecomorphological) traits, physiology, and habitat specialization in Anolis spp. (e.g., Calsbeek et al., 2007; Losos & Sinervo, 1989).
While pairwise interactions of A. sagrei and A. carolinensis have been widely studied elsewhere (Campbell, 2000; Putman et al., 2020; Ryan & Gunderson, 2021), their invasion into the Hawaiian Archipelago offers exciting potential contrasts to prior case studies on species invasions for several reasons: (1) Hawai‘i has no native herpetofauna, but now sustains large populations of both A. sagrei and A. carolinensis introduced in the twentieth century, among other lizard species that were introduced following European contact with Hawai‘i (< 250 years BP), and to a lesser extent by the Polynesians who first colonized Hawai‘i less than 2000 years ago (McKeown, 1996). (2) Among the most common lizards in the archipelago today is an introduced day-gecko from Madagascar (Phelsuma laticauda), which is ecologically similar to A. sagrei and A. carolinensis as a diurnal, arboreal, insectivorous lizard with adhesive toe pads (e.g., Wright et al., 2021). This co-invasion of three ecologically similar lizards presents an exciting opportunity to compare patterns of morphological change among co-invaders as a first step toward identifying the causes and consequences of rapid phenotypic change. Anolis carolinensis, A. sagrei, and Phelsuma laticauda each invaded and spread throughout Hawai‘i’s major islands relatively recently. Anolis carolinensis first appeared in the 1940s following WWII, P. laticauda was intentionally introduced on O‘ahu in the 1970s, while A. sagrei, a common invader across the globe, (e.g., Kolbe et al., 2004, 2007) appeared in the 1980s (McKeown, 1996). Each species’ range expansion into Hawaii has been documented with varying consistency via specimens in natural history collections. These time series provide an opportunity to examine morphological change over the course of each colonization, and test for temporal shifts in ecomorphological traits.
While niche partitioning and subsequent character displacement has been well studied in anoles (Kamath et al., 2020; Muensch et al., 2006), it is unclear how this will manifest in the presence of geckos (Stroud et al., 2019). For example, multiple Phelsuma species have co-invaded Florida (Fieldsend et al., 2021a, b; Meshaka et al., 2004) and now co-occur with both native (A. carolinensis) and introduced anoles (including A. sagrei), but their interactions are not well studied. Dynamics of Phelsuma ecology are generally understudied relative to anoles, although past work has found: (1) habitat partitioning in native ranges (Bungard et al., 2014; Hagey et al., 2016; Harmon et al., 2007; Noble et al., 2011), (2) interactions between introduced and native Phelsuma in the Comoros Islands (Augros et al., 2018), and (3) the introduction of non-native geckos (Hemidactylus frenatus) may impact endemic Phelsuma in Mauritius (Cole & Harris, 2011). Within Hawai‘i, P. laticauda may also be expanding their temporal niche, foraging near artificial lights after dark (Seifan et al., 2010), although these shifts may be more associated with light pollution than niche displacement due to a competing species (Perry et al., 2008; Perry & Fisher, 2006). Regardless of whether ecological shifts in Hawai‘i’s introduced Phelsuma are driven by anoles or other factors, they might be predicted to drive plastic or evolutionary changes in ecomorphology that could be detected by measuring trait change over time.
Herein, we investigate how functional traits change over the course of species invasion for these three introduced lizards (A. sagrei, A. carolinensis, and P. laticauda). We used measurements from both natural history specimens and field-collected individuals from each species to assess ecomorphological variation. While our overarching goal was to compare morphological change among co-invaders, we also generated the following hypotheses concerning potential patterns of change. (1) Based on the dominance of introduced A. sagrei over native A. carolinensis in Florida (Campbell, 2000; Kamath & Stuart, 2015), we predicted that A. carolinensis would exhibit morphological change through time in co-invaded habitats. If A. carolinensis is being driven to higher perches (as seen in Florida) by competitive exclusion from niches at lower heights, we predict that recently collected specimens of this species will possess shorter limbs than counterparts from the earlier invasion stages; this would increase A. carolinensis’ ability to utilize narrower substrates found at greater heights in the understory. (2) Since Hawaiian populations of A. sagrei likely originated from Floridan populations which had adapted to out-compete A. carolinensis (Kolbe et al., 2004), we predict stasis for A. sagrei without ecomorphological change. 3) We hypothesized that P. laticauda’s adhesive adaptations might provide this species with access to habitats unavailable to sympatric invading anoles, and therefore mitigate competition between geckos and anoles within Hawai‘i. Therefore, we might also expect ecomorphological stasis as P. laticauda adapts to the Hawaiian ecosystem. However, the consequence(s) of Anolis-Phelsuma interactions cannot be clearly predicted due to a lack of empirical studies of niche partitioning between these species in field settings.
Methods
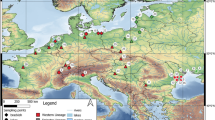
We measured specimens of Anolis carolinensis (N = 25 F, 82 M), A. sagrei (N = 37 F, 67 M), and Phelsuma laticauda (N = 35 F, 19 M); all specimens in Suppl. Table 1. Specimens were collected between 1961 and 2018, most after 1999. Many specimens from the natural history collections were not accompanied by exact geographical coordinates, therefore we did not factor intra-island variation into our analyses. Museum specimens were supplemented by the authors’ field collection in 2018. While some specimens were collected on Kaua‘i in 2018 (Suppl. Table 1) and an effort was made to locate specimens from across the archipelago (specimens found on Hawai‘i, Kaua‘i, Lana‘i, and Māui from UMMZ and MCZ were also measured, but not included in our analyses and are in Suppl. Table 1), the overwhelming majority of our specimens originated from the island of O‘ahu (Fig. 1). Consequently, our subsequent analyses only include collections from O‘ahu as opposed to all the islands.
Sampling localities for all specimens used in this study. Dots indicating sites are not proportional to the number of specimens collected there (see Suppl. Table 1 for complete locality data)
In 2018, lizards were collected by hand or with noose-poles under permit EX-18-09 from the Hawai‘i Department of Natural Land and Resources following IACUC approved protocols (Michigan State University: 04/18-062-00). All samples from 2018 were collected in areas where all three species were observed, and to the best of our knowledge the preserved specimens in this study came from higher trafficked areas of O‘ahu where all three species would likely be present. Animals were euthanized using MS-222 (Conroy et al., 2009), fixed using formalin, and stored in 70% ethanol. Due to concerns over shrinkage in formalin-preserved specimens (Irschick et al., 1997; Losos & de Quieroz, 1997; Vervust et al., 2009, Maayan et al., 2022), live specimens collected in 2018 were measured in 2020, two years after formalin-preservation. To minimize measurement error, all measurements of both museum and field-sampled (then preserved) specimens were collected by a single investigator (JGP). We limited our analyses to adults based on previous work connecting body size (snout vent length or SVL) to sexual maturity (adults in A. carolinensis were defined to have SVL > 40 mm for females and > 45 mm for males (Michael, 1972). For A. sagrei, adults were defined to have > 34 mm SVL for females and > 39 mm for males, (Lee et al., 1989). For P. laticauda, adults were defined with SVL > 43 mm for females and > 40 mm for males (Zug, 2013)).
Morphological measurements were based on characters found to be ecologically relevant in previous studies (Williams, 1983; Losos, 1994). Specifically, the measured traits were snout-vent-length (SVL) and both fore- and hindlimb lengths, measured in segments following previous studies (e.g., Donihue, 2016; Donihue et al., 2018; Hagey et al., 2017; Littleford-Colquhoun et al., 2019; Losos & Miles, 2002) and henceforth using the following abbreviations (see Fig. 2). H1: thigh length (from the point where hindlimb enters the body to apex of knee); H2: crus length (from apex of knee to center of ankle joint); H3: foot length (from center of ankle joint, measured on dorsal side, to tip of the fourth digit i.e. the longest toe including the claw); F1: brachium length (from axilla to apex of elbow joint); F2: antebrachium length (from apex of elbow joint to center of wrist joint, on dorsal side); and F3: hand length (from dorsal center of the wrist joint to tip of longest digit including the claw). For each individual lizard, we summed our forelimb segments (F1 + F2 + F3) to obtain total forelimb length and summed our hindlimb segments (H1 + H2 + H3) to obtain total hindlimb length. All limb measurements were taken on the right side of the body unless damage to toes or the limb prevented consistent measurement (whereupon the left limb was measured). All specimens were used regardless of year collected.
Depiction of morphological features analyzed in this study. SVL (snout-vent-length): distance from the tip of nose to cloaca on ventral side of specimen. F1 (brachium length) distance from axilla to apex of elbow joint. F2 (antebrachium length): distance from apex of elbow joint to center of wrist joint on dorsal side. F3 (hand length): distance from dorsal center of wrist joint to tip of longest digit, including claw. H1 (thigh length): distance from where hindlimb enters body to apex of knee. H2 (crus length): distance from apex of knee to center of ankle joint. H3 (foot length): distance from center of ankle joint (on the dorsal side) to tip of longest digit including claw
All analyses were conducted in R Studio (v. 1.1.463, RStudio Team, 2020) running R version 3.5.3. We first investigated whether it was necessary to partition specimens by sex to calculate residual limb lengths. After natural log transforming our SVL and total fore- and hindlimb length measurements, we used Welch’s two-sample t-test to compare male and female mean SVL (Fig. 3). For each species, for both fore- and hindlimb length, we fit a linear model with limb length as our response variable and SVL as our predictor variable. Residuals from this regression were extracted and we used one-sample, two-sided t-tests to compare male and female residual limb lengths in each species (Fig. 3). These analyses did not support null models in which limb allometries were sexually monomorphic, therefore we analyzed temporal shifts in these traits separately for males and for females in each focal taxon. These results also prompted estimation of fore- and hind- residual limb lengths for each sex (within each species) independently before analyzing morphological change over time. After estimating residual hindlimb lengths for each sex of each species (again using natural log transformed total limb length as a dependent variable on the explanatory variable natural log transformed SVL), we confirmed that our sex-specific limb residuals were normally distributed using Shapiro–Wilk tests.
Preliminary analyses revealed that SVL varies between sex in all three species, with males generally larger than females (A. carolinensis: t(47.5) = − 19.1, p < 0.001; A. sagrei: t(86) = − 14.5, p < 0.001; P. laticauda: t(26) = − 4.69, p < 0.001; Fig. 3). After conducting species-specific regressions of total fore- and hindlimb on SVL and extracting the residuals, we compared mean residual limb lengths of males and females within each species. This approach identifies sex differences in a subset of (residual) limb segments for some (but not all) of the focal species, which led us to analyze sex-specific residual limb lengths for each species (Table 1). We also considered an approach to detect sex-specific differences in species-specific residual limb lengths by comparing mean residual limb lengths of each sex to zero. This approach found similar results, with male and female mean residual fore- and hindlimb lengths in both A. carolinensis and P. laticauda as not significantly departing from zero (p-values > 0.5) suggesting no significant sex-related differences in relative limb length. We conducted two generalized linear mixed models (GLMMs) with total hind limb or total fore limb lengths as our dependent variables, and species and sex as fixed factors and log SVL nested within sex within species. We found similar results as the analyses we present in our paper, with species, sex, and SVL each significantly affecting both fore- and hindlimb lengths, hence justifying our decision to estimate sex and species-specific relative limb lengths for later analyses.
To investigate how SVL and residual limb length may have changed over time, we used both ANCOVAs and estimated Spearman’s r. For each species, we fit a type-2 linear ANCOVA, with either SVL, residual forelimb length, or residual hindlimb length as our dependent variable. Our independent variables included sex and year as well as the interaction between sex and year. We also calculated Spearman’s r for both males and females of each species individually, quantifying the correlation between SVL and year, residual forelimb length and year, and residual hindlimb length and year. Unfortunately, given the nature of sporadic field collections, we were unable to ensure even sampling distribution across years and between sexes. This is a major limitation for many studies that rely on collections and demonstrates a need for more comprehensive sampling over time, especially in common species that are often a priori deemed less worthy of collecting. Since sampling was uneven across species, year, and sex (e.g., no female A. carolinensis in our dataset were collected after 2008), we sought to reduce leverage points where small sample sizes for certain categories might have undue influence over the trends observed. Therefore, outliers and influential data points were detected using Cook’s D (Suppl. Figure 1). We used a threshold value of 1.0 to evaluate if a point had undue leverage on the trend observed (Fox, 2002).
Results
In A. sagrei, mean female hindlimb residual length deviated significantly from zero (t(32) = -2.1504, p = 0.04), while female forelimb length did not (t(32) = -1.75, p = 0.09). Neither A. sagrei male fore- nor hindlimb residual means deviated significantly from zero (fore: t(66) = 1.07, p = 0.3); hind: (t(66) = 1.3, p = 0.2). Together these results also suggest sexual dimorphism in limb length in A. sagrei. Given the associations between sex and limb allometry in the dataset, we used sex-specific residual limb lengths for all three species in the rest of our analyses (see Suppl. Figure 2). We evaluated the normality of these datasets (i.e., sex-specific residual limb lengths) using Shapiro–Wilk tests and found that all 12 sex-specific fore- and hindlimb datasets of residual lengths did not significantly deviate from a normal distribution. See Suppl. Figure 2 for additional data.
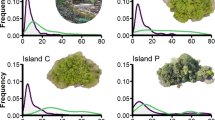
We found sex-specific significant changes in morphology across time for each of our three focal species (Table 2, Fig. 4). Spearman’s r found an increase between SVL and year in A. sagrei males (p = 0.02, r = 0.28), A. carolinensis females (p = 0.02, r = 0.45) and P. laticauda males (p = 0.02, r = 0.52). No species exhibited change in relative forelimb lengths over time (Fig. 4). However, hindlimb length of female A. carolinensis increased over time (Fig. 4). Phelsuma laticauda, A. sagrei, and male A. carolinensis showed no change in hindlimb size over time. However, these results should be interpreted cautiously due to previously mentioned limitations to our dataset. For example, we did not find the same results in male and female A. carolinensis but lacked female individuals from the 2018 field collection. Gaps like these further present the inability to determine if this trend is due to a populational stasis or a limitation of our dataset.
Ecomorphological change in three species of non-native Hawaiian lizards (dark grey = males, light grey = females) obtained from museum and field specimens. SVL (left column), relative forelimb length (center column) and hindlimb length (right column) show sex-specific temporal shifts. Sex-specific linear regressions for traits are shown as lines in each panel with Spearman’s r results are included in each panel
Discussion
Morphological Changes in Hawaii’s Co-invading Lizards?
We found that each of our focal species displayed a different suite of morphological changes in ecologically relevant traits during their co-invasion of Hawai‘i, which were also sex-specific. These rapid morphological changes could have functional consequences for Hawaii’s invasive lizards, given that changes in limb length have great ecological significance in anoles. For example, longer hindlimbs generally confer greater running and jumping abilities on flat surfaces in anoles, whereas shorter hindlimbs typically increase stability when using arboreal perches (Losos & Sinervo, 1989). While we interpret these results cautiously, our findings of morphological changes in Hawai‘i’s invasive A. carolinensis (bigger females with longer hindlimbs) and A. sagrei (bigger males) contrast findings from previous studies elsewhere in these species’ ranges. Following experimental invasions, A. sagrei consistently developed significantly shorter limbs after just two years with no established native species for competition (Kolbe et al., 2012). However, Anolis sagrei also evolved longer limbs (both fore- and hind-) when encountering a different anole invader, A. cristatellus, where both are introduced in Florida, USA (only males measured; Stroud, 2018).
Forelimb length similarly increases in Anolis carolinensis (but shorter hindlimbs) when utilizing perches on smooth leaves than those on branches and tree trunks (Irschick et al., 2005). During the field component of the present study, we often observed A. carolinensis on plants with smooth surfaces (e.g., Pandanus, found in P. laticauda’s native habitat). Unfortunately, we are unable to analyze the perch uses of the museum specimens in this study, and therefore cannot assess whether the morphological changes we observed correspond with historical changes in niche use. Biomechanical aspects of toe lamellae and pads in anoles and geckos are likely at play in this ecosystem (Winchell et al., 2018). When considering our observed shifts in A. carolinensis and A. sagrei morphology in light of previous work involving these species, there are two main factors that could make the Hawaiian anoles different.
(1) Previous studies have focused on invasive A. sagrei interacting with native A. carolinensis, while in Hawai‘i, both species are introduced. Therefore, one possibility in our study is that colonizing A. carolinensis may have undergone a bottleneck and/or selection for ‘invasive’ traits whereas native populations would have greater genetic variation and would be already well-adapted to Hawai‘i. (2) Invasion pathways could also have had important impacts on our findings concerning A. sagrei. A species-wide analysis of this species found that one Hawaiian population contained haplotypes most similar to invasive populations in Florida, rather than haplotypes found within their native Cuba (Kolbe et al., 2004). This suggests that the original A. sagrei invaders to Hawai‘i were in contact with native A. carolinensis populations prior to their arrival. Anolis carolinensis in Hawai‘i appear to have resulted from at least two introductions from the southern US (excepting peninsular Florida) and are distantly related to other invasive Pacific populations in Okinawa and the Ogasawara Islands (Suzuki-Ohno et al., 2017).
While no one has yet examined fine-scale genetic diversity in Hawaiian Phelsuma laticauda, our observed results may be due to a genetic bottleneck. We suspect this for three reasons: (1) Hawaiian P. laticauda are believed to have stemmed from one intentional introduction of eight individuals outside of Honolulu in 1974 (McKeown, 1996). While P. laticauda has been successfully introduced into other non-native habitats (Kraus, 2009), The first records of introduced P. laticauda other than Hawai‘i were the Comoros in the late 1990s (Meirte, 1999) and French Polynesia in 2006 (Ota & Ineich, 2006). Therefore, we are reasonably confident that this founder population originated through the pet trade, originally from Madagascar. Without additional introduction events, Hawai‘i’s P. laticauda would have experienced a significant bottleneck which may have constrained diversification and local adaptation. (2) Allometric studies of geckos in conjunction with rapid evolution have not been nearly as widespread as in anoles, so rates of adaptation are poorly understood in comparison to anoles. Therefore, the amount of time needed for morphological shifts to manifest may not yet have passed. 3) Finally, P. laticauda may be behaviorally dominant to both anole species and is not displaced from its expected native niche when interacting with A. carolinensis and A. sagrei.
The second unique feature of our Hawaiian (co-invasion) case study is the presence of P. laticauda, which routinely appeared at higher perches than either anole in the field (pers obs). Specifically, we found that male P. laticauda forelimb lengths increased over time. The drivers and ecological impacts of this change are challenging to infer due to the lack of prior studies and context. While anole ecomorphology has been widely studied outside of Hawaii, it is unknown how morphological shifts, such as the observed forelimb changes, impact Phelsuma performance or niche use (but see Wright et al. (2021) on habitat shifts in experimental enclosures). If the biomechanical and ecomorphological relationships reported from Caribbean anoles can be applied to Phelsuma, then the patterns described above could be indicative of adaptation to novel selective pressures (e.g., perches and textures and/or habitat partitioning with novel competitors in the invaded ecosystem). We observed antagonistic behaviors between Phelsuma and anoles during our fieldwork, although other studies found low rates of interspecific aggression among Hawaiian anoles (Kennedy-Gold, 2019). Since past work suggested native A. carolinensis shift higher in trees in the presence of invasive A. sagrei, the presence of another high-perched lizard (P. laticauda) may have prevented this from occurring within Hawai‘i. Furthermore, the order in which communities assemble can influence downstream interactions (priority effect; MacArthur, 1972, or incumbency Fukami, 2015). If A. carolinensis were the first of these species to colonize Hawai‘i (as is widely believed), they may have acclimated/adapted well in advance of Phelsuma or A. sagrei giving them a distinct toe(pad) hold.
In this work, we consider both anole species to be invasive within Hawai‘i due to their widespread abundance and documented impacts on other novel ecosystems—predominantly due to predation and secondary trophic effects (Kraus, 2009 and sources within). The detrimental impacts of introduced P. laticauda have not yet been demonstrated in Hawai‘i, but Hawai‘i’s Department of Land and Natural Resources lists all species of Phelsuma as “injurious wildlife” (dlnr.hawaii.gov), and the species occurs throughout much of the archipelago. In the Comoros Islands, introduced P. laticauda appear to be dominant to native species of geckos (Carretero et al., 2005). However, as there are no native lizards to the Hawaiian Islands its designation is somewhat unclear in this respect.
Below we discuss the difficulties of disentangling phenotypic plasticity and selection as drivers of the patterns reported herein. In principle, however, evolutionary changes (perhaps driven by interspecies interactions) might underlie some of our findings. The bulk of our specimens were collected recently (1999–2018), comparable to the 20-generation span over which Stuart et al. (2014) found significant morphological changes in A. sagrei (although they looked at toepad size, which likely evolves at a different tempo that SVL and limb length). However, to determine the presence or magnitude of selection, we would need to incorporate future studies with genomic evidence, common garden experiments or both (de Villemereuil et al., 2016; Winchell et al., 2023). Importantly, these data only allow us to confidently evaluate morphological shifts that have occurred more recently as opposed to across the entire invasion of each species. Future work will benefit from the documentation of specimens during the earliest stages of a species’ colonization, and we encourage curators to voucher specimens of non-native species during their earliest known occurrences in novel ecosystems. More comprehensive collections, together with genetic analyses, would help illuminate how plasticity and evolution contribute to invasion dynamics during each phase of staggered co-invasions by species with overlapping niches (Fieldsend et al., 2021a, 2021b; Kolbe et al., 2004, 2007).
Our 2018 field collection on Kaua‘i produced nine additional specimens (six male, three female) which had longer limbs than O‘ahu or Hawai‘i. It is noteworthy that these data suggest morphological differences between P. laticauda on different islands within Hawai‘i (Suppl. Table 1). This finding warrants further study and we omitted the Kaua‘i specimens (as well as other non-O‘ahu samples) in our analyses due to insufficient sample sizes and limited availability of museum specimens for this species. Sample sizes at either end of our time span (both pre-1999 and 2008–2018) are minimal, which may have placed undue leverage on these datapoints in driving the results of our analyses. This further illustrates the need for consistent collection through time of invaders such as these, and robust series in natural history collections can shed more light on the dynamics of evolution in a changing environment.
Potential (Co-)drivers of Morphological Change
We suggest that competition among co-invading lizard taxa, and/or species- and sex-specific adaptations to the Hawaiian ecosystem might have driven the shifts in morphology that we report herein. However, some changes may also result from selection for dispersal. Calsbeek et al. (2009) found that small A. sagrei males disperse more often than larger conspecifics as dispersal behavior likely reduces male-male competition for territories. Selection for dispersal-related traits has been observed in Australia’s introduced cane toads, which evolved longer legs and other traits at the leading edge of their introduced range (Phillips et al., 2006, 2010). In cane toads, dispersal reduces resource competition due to higher abundance in novel prey at the invasion front (Brown et al., 2013).
Many local Hawaiians claimed to observe the brown anoles displacing green anoles, although the layman’s difficulty to distinguish between a brown A. carolinensis and an A. sagrei may limit the reliability of these reports. Furthermore, if A. sagrei were displacing A. carolinensis to higher perches, as seen in the southeastern United States (Edwards & Lailvaux, 2012; Stuart et al., 2014; Borden et al., 2022), this may make A. carolinensis less visible to the observer even if they remained present at unchanging density. Studies have also demonstrated that dispersal by A. carolinensis to novel islands is inhibited (if not prevented) by co-invading of A. sagrei (Losos & Spiller, 1999) but given A. carolinensis’ earlier arrival in Hawai‘i, these phenomena may not be relevant in this system.
Predation and Abiotic Selection as Other Potential Drivers of Morphological Change
Another factor driving our observed patterns in Hawaiian lizards could be a novel suite of predators. Shifts in limb length have been demonstrated in A. sagrei over a single generation in response to a predatory co-invasive lizard (Leiocephalus carinatus) along with a shift to higher perches (Losos et al., 2006). Previous studies have identified domestic/feral cats, basilisks, and whiptail lizards (Ameiva exsul) as potential predators of Florida anoles (Avilés-Rodríguez & Kolbe, 2019). Hawai‘i lacks any native lizards that could represent a threat, although it does have large numbers of feral cats, rats, and mongooses (pers. obs, Hess et al., 2007; Loope et al., 1988) some of which have been documented to consume lizards (species unidentified, Hill et al., 2019; Mostello & Conant, 2018). There are a few introduced lizards that dwarf our focal species and may serve as potential predators. In particular, the Jackson’s Chameleon (Trioceros jacksonii), which has been highly problematic for endemic land snails (Chiaverano & Holland, 2014), is estimated to occur in high densities on at least one island (Kraus et al., 2012). However, we did not observe this species in our sampling sites as it generally occupies higher elevation, high canopy sites (Chiaverano & Holland, 2014). Hawai‘i is also home to a suite of invasive birds, some of which (e.g., mynahs and chickens) readily prey upon lizards in other ecosystems (Oliver & Shaw, 1953; Cisneros-Heredía 2018; pers. obs). One example of a native owl consuming an unknown lizard was also found (Mostello & Conant, 2018). Another variable unaccounted for is tropical storms in the Pacific, which likely reflect additional selection forces (independent of competition between the co-invaders). Hurricanes can cause morphological change in Caribbean anoles (Donihue et al., 2020; Rabe et al., 2020) and are relatively frequent in the Hawaiian Islands. The flora of Hawai‘i also represent a heterogeneous mixture of native and non-native plants—from both within and beyond the native ranges of our focal lizard species.
Conclusions
Species invasions are frequently viewed through a negative lens, yet they provide ideal natural experiments to understand ecological shifts within colonizing populations. Our work demonstrates how museum specimens of invasive taxa can help illuminate these processes and highlights the importance of collecting and preserving non-native specimens through all stages of invasion in service of future, retrospective case studies. This is an important message for biologists to broadcast among collectors and curators. Because biodiversity collection efforts are chiefly focused on native species, some collectors might not appreciate the potential utility of replicated sampling of invasives over space and/or through time.
By comparing museum and contemporary specimens from Hawai‘i we found distinctive patterns of morphological change over time among three species of arboreal, diurnal lizards. Based on prior research, we suggest that these may reflect outcomes of species- and sex-specific selection pressures, some of which result from interactions among the co-invading species. Testing of these scenarios awaits detailed field and/or experimental studies (e.g., Wright et al., 2021) that will be essential in advancing our understanding of how colonizing taxa interactively shape invasion outcomes.
Data Availability
All raw data can be found in the supplement for this paper.
References
Augros, S., Scherz, M. D., Wang-Claypool, C. Y., Montfort, L., Glaw, F., & Hawlitschek, O. (2018). Comparative perch heights and habitat plant usage of day geckos (Phelsuma) in the Comoros Archipelago (Squamata: Gekkonidae). Salamandra, 54(1), 71–74.
Avilés-Rodríguez, K. J., & Kolbe, J. J. (2019). Escape in the city: Urbanization alters the escape behavior of Anolis lizards. Urban Ecosystems, 22, 733–742. https://doi.org/10.1007/s11252-019-00845-x
Bellard, C., Cassey, P., & Blackburn, T. M. (2016). Alien species as a driver of recent extinctions. Biology Letters, 12(4), 20150623. https://doi.org/10.1098/rsbl.2015.0623
Borden, J. B., Bohlman, S., & Scheffers, B. R. (2022). Niche lability mitigates the impact of invasion but not urbanization. Oecologia, 198(1), 1–10. https://doi.org/10.1007/s00442-021-05039-x
Brown, G. P., Kelehear, C., & Shine, R. (2013). The early toad gets the worm: Cane toads at an invasion front benefit from higher prey availability. Journal of Animal Ecology, 82(4), 854–862. https://doi.org/10.1111/1365-2656.12048
Bungard, M. J., Jones, C. G., Tatayah, V., & Bell, D. J. (2014). The habitat use of two species of day geckos (Phelsuma ornata and Phelsuma guimbeaui) and implications for conservation management in island ecosystems. Herpetological Conservation and Biology, 9(3), 551–562.
Calsbeek, R., Bonvini, L., & Cox, R. M. (2009). Geographic variation, frequency-dependent selection, and the maintenance of a female-limited polymorphism. Evolution, 64(1), 116–125. https://doi.org/10.1111/j.1558-5646.2009.00808.x
Calsbeek, R., Smith, T. B., & Bardeleben, C. (2007). Intraspecific variation in Anolis sagrei mirrors the adaptive radiation of Greater Antillean anoles. Biological Journal of the Linnean Society, 90(2), 189–199. https://doi.org/10.1111/j.1095-8312.2007.00700.x
Campbell, T. S. (2000). Analyses of the effects of an exotic lizard (Anolis sagrei) on a native lizard (Anolis carolinensis) in Florida, using islands as experimental units. University of Tennessee.
Capinha, C., Marcolin, F., & Reino, L. (2020). Human-induced globalization of insular herpetofaunas. Global Ecology and Biogeography, 29, 1328–1349. https://doi.org/10.1111/geb.13109
Carretero, M. A., Harris, D. J., & Rocha, S. (2005). Recent observations of reptiles in the Comoro islands (Western Indian Ocean). Herpetological Bulletin, 91, 19–28.
Chapple, D. G., Miller, K. A., Kraus, F., & Thompson, M. B. (2013). Divergent introduction histories among invasive populations of the delicate skink (Lampropholis delicata): Has the importance of genetic admixture in the success of biological invasions been overemphasized? Diversity and Distributions, 19(2), 134–146. https://doi.org/10.1111/j.1472-4642.2012.00919.x
Chiaverano, L. M., & Holland, B. S. (2014). Impact of an invasive predatory lizard on the endangered Hawaiian tree snail Achatinella mustelina: A threat assessment. Endangered Species Research, 24(2), 115–123. https://doi.org/10.3354/esr00589
Cisneros-Heredia, D. F. (2018). The hitchhiker wave: non-native small terrestrial vertebrates in the Galápagos. In Understanding Invasive Species in the Galapagos Islands (pp. 95–139). Springer International Publishing. https://doi.org/10.1007/978-3-319-67177-2_7
Cole, N. C., & Harris, S. (2011). Environmentally-induced shifts in behavior intensify indirect competition by an invasive gecko in Mauritius. Biological Invasions, 13(9), 2063–2075. https://doi.org/10.1007/s10530-011-0025-8
Conroy, C. J., Papenfuss, T., Parker, J., & Hahn, N. E. (2009). Use of tricaine methanesulfonate (MS222) for euthanasia of reptiles. Journal of the American Association for Laboratory Animal Science, 48(1), 28–32.
de Villemereuil, P., Gaggiotti, O. E., Mouterde, M., & Till-Bottraud, I. (2016). Common garden experiments in the genomic era: New perspectives and opportunities. Heredity, 116(3), 249–254. https://doi.org/10.1038/hdy.2015.93
Donihue, C. (2016). Microgeographic variation in locomotor traits among lizards in a human-built environment. PeerJ, 4, e1776. https://doi.org/10.7717/peerj.1776
Donihue, C. M., Herrel, A., Fabre, A. C., Kamath, A., Geneva, A. J., Schoener, T. W., et al. (2018). Hurricane-induced selection on the morphology of an island lizard. Nature, 560(7716), 88–91. https://doi.org/10.1038/s41586-018-0352-3
Donihue, C. M., Kowaleski, A. M., Losos, J. B., Algar, A. C., Baeckens, S., Buchkowski, R. W., et al. (2020). Hurricane effects on Neotropical lizards span geographic and phylogenetic scales. Proceedings of the National Academy of Sciences of the United States of America, 117(19), 10429–10434. https://doi.org/10.1073/pnas.2000801117
Edwards, J. R., & Lailvaux, S. P. (2012). Display behavior and habitat use in single and mixed populations of Anolis carolinensis and Anolis sagrei lizards. Ethology, 118(5), 494–502. https://doi.org/10.1111/j.1439-0310.2012.02037.x
Fieldsend, T. W., Dubos, N., Krysko, K. L., Raxworthy, C. J., & Malone, S. L. (2021a). In situ adaptation and ecological release facilitate the occupied niche expansion of a non-native reptile. Ecology and Evolution, 11, 9410–9422. https://doi.org/10.1002/ece3.7749
Fieldsend, T. W., Krysko, K. L., Sharp, P., & Collins, T. M. (2021b). Provenance and genetic diversity of the non-native geckos Phelsuma grandis Gray 1870 and Gekko gecko (Linnaeus 1758) in southern Florida, USA. Biological Invasions, 23(5), 1649–1662. https://doi.org/10.1007/s10530-021-02463-1
Fox, J. (2002). An R and S-Plus companion to applied Regression. Sage.
Fukami, T. (2015). Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annual Review of Ecology, Evolution, and Systematics, 46, 1–23. https://doi.org/10.1146/annurev-ecolsys-110411-160340
Gallardo, B., Bacher, S., Bradley, B., Comín, F. A., Gallien, L., Jeschke, J. M., et al. (2019). InvasiBES: Understanding and managing the impacts of Invasive alien species on Biodiversity and Ecosystem Services. NeoBiota, 50(2019), 109–122. https://doi.org/10.3897/neobiota.50.35466
Grosholz, E. D. (2005). Recent biological invasion may hasten invasional meltdown by accelerating historical introductions. Proceedings of the National Academy of Sciences of the United States of America, 102(4), 1088–1091. https://doi.org/10.1073/pnas.0308547102
Hagey, T. J., Cole, N. C., Davidson, D., Henricks, A., Harmon, L. L., & Harmon, L. J. (2016). Temporal variation in structural microhabitat use of Phelsuma Geckos in Mauritius. Journal of Herpetology, 50(1), 102–107. https://doi.org/10.1670/13-136
Hagey, T. J., Harte, S., Vickers, M., Harmon, L. J., & Schwarzkopf, L. (2017). There’s more than one way to climb a tree: Limb length and microhabitat use in lizards with toe pads. PLoS ONE, 12(9), e0184641. https://doi.org/10.1371/journal.pone.0184641
Harmon, L. J., Harmon, L. L., & Jones, C. G. (2007). Competition and community structure in diurnal arboreal geckos (genus Phelsuma) in the Indian Ocean. Oikos, 116(11), 1863–1878. https://doi.org/10.1111/j.2007.0030-1299.15958.x
Hess, S. C., Hansen, H., Nelson, D., Swift, R., & Banko, P. C. (2007). Diet of feral cats in Hawai‘i Volcanoes National Park. Pacific Conservation Biology, 13(4), 244–249. https://doi.org/10.1071/pc070244
Hill, S. A., Beard, K. H., Siers, S. R., & Shiels, A. B. (2019). Invasive coqui frogs are associated with differences in mongoose and rat abundances and diets in Hawai‘i. Biological Invasions, 21(6), 2177–2190. https://doi.org/10.1007/s10530-019-01965-3
Irschick, D. J., Carlisle, E., Elstrott, J., Ramos, M., Buckley, C., Vanhooydonck, B., et al. (2005). A comparison of habitat use, morphology, clinging performance and escape behaviour among two divergent green anole lizard (Anolis carolinensis) populations. Biological Journal of the Linnean Society, 85(2), 223–234. https://doi.org/10.1111/j.1095-8312.2005.00487.x
Irschick, D. J., Vitt, L. J., Zani, P. A., & Losos, J. B. (1997). A comparison of evolutionary radiations in mainland and Caribbean Anolis lizards. Ecology, 78(7), 2191–2203.
Jackman, T. R., Larson, A., & de Queiroz, K. (1999). Phylogenetic relationships and tempo of early diversification in Anolis lizards. Systematic Biology, 48(2), 254–285.
Jackson, M. C. (2015). Interactions among multiple invasive animals. Ecology, 96(8), 2035–2041.
Kamath, A., & Stuart, Y. E. (2015). Movement rates of the lizard Anolis carolinensis (Squamata: Dactyloidae) in the presence and absence of Anolis sagrei (Squamata: Dactyloidae). Breviora, 546, 1–7.
Kamath, A., Herrmann, N. C., Gotanda, K. M., Shim, K. C., LaFond, J., Cottone, G., et al. (2020). Character displacement in the midst of background evolution in island populations of Anolis lizards: A spatiotemporal perspective. Evolution, 74(10), 2250–2264. https://doi.org/10.1111/evo.14079
Kennedy-Gold, S. (2019). Indirect and direct effects of competitor presence on behavior of introduced anoles in Hawai‘i. University of Hawai‘i at Manoa.
Kolbe, J. J., Glor, R. E., Rodríguez Schettino, L., Lara, A. C., Larson, A., & Losos, J. B. (2004). Genetic variation increases during biological invasion by a Cuban lizard. Nature, 431(7005), 177–181. https://doi.org/10.1038/nature02807
Kolbe, J. J., Larson, A., & Losos, J. B. (2007). Differential admixture shapes morphological variation among invasive populations of the lizard Anolis sagrei. Molecular Ecology, 16(8), 1579–1591. https://doi.org/10.1111/j.1365-294X.2006.03135.x
Kolbe, J. J., & Losos, J. B. (2005). Hind-limb length plasticity in Anolis carolinensis. Journal of Herpetology, 39(4), 674–678.
Kolbe, J. J., Schoener, T. W., Spiller, D. A., & Losos, J. B. (2012). Founder effects persist despite adaptive differentiation: A field experiment with lizards. Science, 335, 1086–1089.
Kraus, F. (2009). Alien reptiles and amphibians, a scientific compendium and analysis. Springer. https://doi.org/10.1111/ppa.12538
Kraus, F., Medeiros, A., Preston, D., Jarnevich, C. S., & Rodda, G. H. (2012). Diet and conservation implications of an invasive chameleon, Chamaeleo jacksonii (Squamata: Chamaeleonidae) in Hawaii. Biological Invasions, 14, 579–593.
Lee, J. C., Clayton, D., Eisenstein, S., & Perez, I. (1989). The reproductive cycle of Anolis sagrei in southern Florida. Copeia, 1989(4), 930–937. https://doi.org/10.2307/1445979
Littleford-Colquhoun, B. L., Clemente, C., Thompson, G., Cristescu, R. H., Peterson, N., Strickland, K., et al. (2019). How sexual and natural selection shape sexual size dimorphism: Evidence from multiple evolutionary scales. Functional Ecology, 33(8), 1446–1458. https://doi.org/10.1111/1365-2435.13337
Llewelyn, J., Phillips, B. L., Brown, G. P., Schwarzkopf, L., Alford, R. A., & Shine, R. (2011). Adaptation or preadaptation: Why are keelback snakes (Tropidonophis mairii) less vulnerable to invasive cane toads (Bufo marinus) than are other Australian snakes? Evolutionary Ecology, 25(1), 13–24. https://doi.org/10.1007/s10682-010-9369-2
Loope, L. L., Hamann, O., & Stone, C. P. (1988). Comparative conservation biology of oceanic archipelagoes: Hawai‘i and the Galápagos. BioScience, 38(4), 272–282.
Losos, J. B. (1994). An approach to the analysis of comparative data when a phylogeny is unavailable or incomplete. Systematic Biology, 43(1), 117–123.
Losos, J. B., & Spiller, D. A. (1999). Differential colonization success and asymmetrical interactions between two lizard species. Ecology, 80(1), 252–258. https://doi.org/10.1890/0012-9658(1999)080[0252:DCSAAI]2.0.CO;2
Losos, J. B. (2009). Lizards in an evolutionary tree: Ecology and adaptive radiation of anoles. University of California Press.
Losos, J. B., Creer, D. A., Glossip, D., Goellner, R., Hampton, A., Roberts, G., et al. (2000). Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution, 54(1), 301–305.
Losos, J. B., & de Queiroz, K. (1997). Evolutionary consequences of ecological release in Caribbean Anolis lizards. Biological Journal of the Linnean Society, 61(4), 459–483. https://doi.org/10.1006/bijl.1997.0137
Losos, J. B., Glor, R. E., Kolbe, J. J., & Nicholson, K. E. (2006). Adaptation, speciation, and convergence: A hierarchical analysis of adaptive radiation in Caribbean Anolis lizards. Annals of the Missouri Botanical Garden, 93, 24–33.
Losos, J. B., & Miles, D. B. (2002). Testing the hypothesis that a clade has adaptively radiated: Iguanid lizard clades as a case study. The American Naturalist, 160(2), 147–157.
Losos, J. B., & Sinervo, B. (1989). The effects of morphology and perch diameter on sprint performance of Anolis lizards. Journal of Experimental Biology, 30, 23–30.
Losos, J. B., Warheit, K. I., & Schoener, T. W. (1997). Adaptive differentiation following experimental island colonization in Anolis lizards. Nature, 387, 70–73.
Maayan, I., Reynolds, R. G., Goodman, R. M., Hime, P. M., Bickel, R., Luck, E. A., & Losos, J. B. (2022). Fixation and preservation contribute to distortion in vertebrate museum specimens: A 10-year study with the lizard Anolis sagrei. Biological Journal of the Linnean Society, 136(3), 443–454. https://doi.org/10.1093/biolinnean/blac040
MacArthur, R. H. (1972). Geographical ecology. Harper and Row.
McKeown, S. (1996). A field guide to reptiles and amphibians in the Hawaiian Islands. Diamond Head Publishing Inc.
Meirte, D. (1999). Reptiles. Annales Du Musée Royale De L’afrique Centrale, Sciences Zoologique, 284, 114–132.
Meshaka, W. E., Jr., Butterfield, B. P., & Hauge, J. B. (2004). The exotic amphibians and reptiles of Florida. Krieger Publishing.
Michael, E. D. (1972). Growth rates in Anolis carolinensis. Copeia, 1972(3), 575–577. https://doi.org/10.2307/1442932
Mooney, H. A., & Cleland, E. E. (2001). The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America, 98(10), 5446–5451. https://doi.org/10.1073/pnas.091093398
Mostello, C. S., & Conant, S. (2018). Diets of native and introduced apex predators in Hawai‘i. Pacific Conservation Biology, 24(1), 25–34. https://doi.org/10.1071/PC17042
Mothes, C. C., Stroud, J. T., Clements, S. L., & Searcy, C. A. (2019). Predicting the invasion dynamics of anoles (and other lizards) using ecological niche modeling. Anolis Newsletter VII, 194–205. https://doi.org/10.7936/gjg3-h168
Muensch, J., Leininger, P. D., Werth, D. E., Fawks, A. M., & Thomas, S. M. (2006). The anoles of Coconut Island, Kane’ohe Bay, O’ahu. Hawai‘i. Iguana, 13(3), 199–205.
Noble, T., Bunbury, N., Kaiser-Bunbury, C. N., & Bell, D. J. (2011). Ecology and co-existence of two endemic day gecko (Phelsuma) species in Seychelles native palm forest. Journal of Zoology, 283(1), 73–80. https://doi.org/10.1111/j.1469-7998.2010.00751.x
Oliver, J. A., & Shaw, C. E. (1953). The amphibians and reptiles of the Hawaiian Islands. Zoologica, 38(5), 65–95. https://doi.org/10.1007/978-94-009-6539-3_20
Ota, H., & Ineich, I. (2006). Colonization of the gold dust day gecko, Phelsuma laticauda (Reptilia: Gekkonidae), in Moorea of the Society Archipelago, French Polynesia. Current Herpetology, 25, 97–99.
Perry, G., Buchanan, B. W., Fisher, R. N., Salmon, M., & Wise, S. E. (2008). Effects of artificial night lighting on amphibians and reptiles in urban environments. In J. C. Mitchell, R. E. Jung Brown, & B. Bartholomew (Eds.), Urban herpetology (pp. 239–256). Society for the Study of Amphibians and Reptiles.
Perry, G., & Fisher, R. N. (2006). Night lights and reptiles: Observed and potential effects. In C. Rich & T. Longcore (Eds.), Ecological consequences of artificial night lighting (pp. 169–191). Island Press.
Phillips, B. L., Brown, G. P., & Shine, R. (2010). Evolutionarily accelerated invasions: The rate of dispersal evolves upwards during the range advance of cane toads. Journal of Evolutionary Biology, 23(12), 2595–2601. https://doi.org/10.1111/j.1420-9101.2010.02118.x
Phillips, B. L., Brown, G. P., Webb, J. K., & Shine, R. (2006). Invasion and the evolution of speed in toads. Nature, 439(7078), 803–803. https://doi.org/10.1038/439803a
Pringle, R. M., Kartzinel, T. R., Palmer, T. M., Thurman, T. J., Fox-Dobbs, K., Xu, C. C. Y., et al. (2019). Predator-induced collapse of niche structure and species coexistence. Nature, 570(7759), 58–64. https://doi.org/10.1038/s41586-019-1264-6
Putman, B. J., Pauly, G. B., & Blumstein, D. T. (2020). Urban invaders are not bold risk-takers: A study of 3 invasive lizards in southern California. Current Zoology, 66(6), 657–665. https://doi.org/10.1093/CZ/ZOAA015
R Core Team. (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Rabe, A. M., Herrmann, N. C., Culbertson, K. A., Donihue, C. M., & Prado-Irwin, S. R. (2020). Post-hurricane shifts in the morphology of island lizards. Biological Journal of the Linnean Society, 130(1), 156–165. https://doi.org/10.1093/biolinnean/blaa022
Rius, M., & Darling, J. A. (2014). How important is intraspecific genetic admixture to the success of colonising populations? Trends in Ecology & Evolution, 29(4), 233–242. https://doi.org/10.1016/j.tree.2014.02.003
RStudio: Integrated Development for R. (2020). Integrated development for R. RStudio, PBC.
Ryan, L. M., & Gunderson, A. R. (2021). Competing native and invasive Anolis lizards exhibit thermal preference plasticity in opposite directions. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 335(1), 118–125. https://doi.org/10.1002/jez.2420
Seifan, T., Federman, A., Mautz, W. J., Smith, K. J., & Werner, Y. L. (2010). Nocturnal foraging in a diurnal tropical lizard (Squamata: Gekkonidae: Phelsuma laticauda) on Hawai‘i. Journal of Tropical Ecology, 26(2), 243–246. https://doi.org/10.1017/S0266467409990484
Sillero, N., Huey, R. B., Gilchrist, G. W., Rissler, L. J., & Pascual, M. (2020). Distribution modelling of an introduced species: Do adaptive genetic markers affect potential range? Proceedings of the Royal Society B: Biological Sciences, 287, 20201791.
Stroud, J. T. (2018). Using introduced species of Anolis lizards to test adaptive radiation theory. Florida International University.
Stroud, J. T., Geneva, A. J., & Losos, J. B. (Eds.). (2019). Anolis Newsletter VII. Washington University, St. Louis MO.
Stroud, J. T., Mothes, C. C., Beckles, W., Heathcote, R. J. P., Donihue, C. M., & Losos, J. B. (2020). An extreme cold event leads to community-wide convergence in lower temperature tolerance in a lizard community: Convergent shifts in extreme events. Biology Letters, 16(10), 20200625. https://doi.org/10.1098/rsbl.2020.0625rsbl20200625
Stuart, Y. E., Hohenlohe, P. A., Reynolds, R. G., Revell, L. J., Losos, J. B., & Campbell, T. S. (2014). Rapid evolution of a native species following invasion by a congener. Science, 346(6208), 463–466. https://doi.org/10.1126/science.1257008
Suzuki-Ohno, Y., Morita, K., Nagata, N., Mori, H., Abe, S., Makino, T., & Kawata, M. (2017). Factors restricting the range expansion of the invasive green anole Anolis carolinensis on Okinawa Island Japan. Ecology and Evolution, 7(12), 4357–4366. https://doi.org/10.1002/ece3.3002
Tamate, S., Iwasaki, W. M., Krysko, K. L., Camposano, B. J., Mori, H., Funayama, R., et al. (2017). Inferring evolutionary responses of Anolis carolinensis introduced into the Ogasawara Archipelago using whole genome sequence data. Scientific Reports, 7, 18008. https://doi.org/10.1038/s41598-017-17852-7
Vervust, B., van Dongen, S., & Van Damme, R. (2009). The effect of preservation on lizard morphometrics – An experimental study. Amphibia-Reptilia, 30(3), 321–329. https://doi.org/10.1163/156853809788795209
Wehsener, J. W., & Noss, C. F. (2022). Foraging mode and the factors affecting foraging behavior in the diurnal arboreal gecko, Phelsuma laticauda. Journal of Herpetology, 56(4), 386–395.
Williams, E. E. (1983). Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. In Lizard ecology (pp. 326–370).
Winchell, K. M., Campbell-Staton, S. C., Losos, J. B., Revell, L. J., Verrelli, B. C., & Geneva, A. J. (2023). Genome-wide parallelism underlies contemporary adaptation in urban lizards. Proceedings of the National Academy of Sciences, 120(3), e2216789120. https://doi.org/10.1073/pnas
Winchell, K. M., Maayan, I., Fredette, J. R., & Revell, L. J. (2018). Linking locomotor performance to morphological shifts in urban lizards. Proceedings of the Royal Society b: Biological Sciences, 285, 20180229. https://doi.org/10.1098/rspb.2018.0229
Winchell, K. M., Reynolds, R. G., Prado-Irwin, S. R., Puente-Rolón, A. R., & Revell, L. J. (2016). Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution, 70(5), 1009–1022. https://doi.org/10.1111/evo.12925
Wright, A. N., Kennedy-Gold, S., Naylor, E. R., Screen, R. M., Piantoni, C., & Higham, T. E. (2021). Clinging performance on natural substrates predicts habitat use in anoles and geckos. Functional Ecology, 35, 2472–2482. https://doi.org/10.1111/1365-2435.13919
Yoder, J. B., Clancey, E., Des Roches, S., Eastman, J. M., Gentry, L., Godsoe, W., et al. (2010). Ecological opportunity and the origin of adaptive radiations. Journal of Evolutionary Biology, 23(8), 1581–1596. https://doi.org/10.1111/j.1420-9101.2010.02029.x
Yuan, M. L., Jung, C., Wake, M. H., & Wang, I. J. (2020). Habitat use, interspecific competition and phylogenetic history shape the evolution of claw and toepad morphology in Lesser Antillean anoles. Biological Journal of the Linnean Society, 129(3), 630–643. https://doi.org/10.1093/biolinnean/blz203
Zug, G. R. (2013). Reptiles and amphibians of the Pacific Islands: A comprehensive guide. University of California Press. https://doi.org/10.5860/choice.51-2090
Acknowledgements
We are indebted to Greg Schneider at UMMZ, Stevie Kennedy-Gold at MCZ and Brad Hollingsworth at SDNHM for access to and assistance with the preserved specimens that allowed us to conduct these analyses. We also thank David Smith for permitting assistance to collect specimens in the field as well as Tobias Koehler and Valerie Puni at the National Tropical Botanical Garden, Richard Pezzulo and Josie Hoh at Waimea Valley and Joshlyn Sand at the Honolulu Botanical Garden for access to collection of live specimens. Louise Mead assisted in obtaining IACUC approval. J. Deitloff and members of the Parent and Harmon labs provided helpful discussion on earlier drafts of this manuscript. We also thank J. Kolbe and several anonymous reviewers for providing critical feedback on this manuscript.
Funding
This work was partially supported by travel funds through both the BEACON Center and Michigan State’s EEBB program (JGP, TJH, EJG).
Author information
Authors and Affiliations
Contributions
JGP, TJH, and EJG contributed to the study conception and design. All authors contributed to material preparation, data collection and analysis. The first draft of the manuscript was written by JGP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Phillips, J.G., Hagey, T.J., Hagemann, M. et al. Analysis of Morphological Change during a Co-invading Assemblage of Lizards in the Hawaiian Islands. Evol Biol 51, 257–268 (2024). https://doi.org/10.1007/s11692-024-09631-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-024-09631-w