Abstract
Phenotypic variability is a key factor promoting the establishment and spread of invasive populations in new environments. The Ponto-Caspian region contains a diverse endemic fauna known for its exceptional environmental plasticity, with many species invading European waters. However, the extent to which the environment shapes the phenotypic variability of these successful invaders remains poorly understood. We test to what extent the environment, intraspecific lineage affinity and geographic range interact and shape the variability of ecologically relevant functional morphological traits of the amphipod, Dikerogammarus villosus. Our results show the highest differentiation among environments, with an enhancement of predation-related traits in brackish waters relative to freshwaters. Differentiation among lineages and ranges (native/invaded) was smaller, occurring in traits related to locomotion and food processing. Although we uncovered an overall increase in variability outside the native range, the dynamics of morphological change were lineage-specific: the Western Lineage (invading via the River Danube) underwent a shift towards increased appendage length, while the Eastern Lineage (invading via the River Dnieper) underwent a significant overall morphospace expansion. We conclude that D. villosus exhibits a remarkable morphological variability across Europe that is influenced by the interplay between the environment as well as its evolutionary and invasion history.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-native organisms often encounter various novel selection pressures that drive their evolutionary adaptation in the invaded range (Suarez & Tsutsui, 2008; Atwood & Meyerson, 2011). Invasive species in recently colonised habitats need to adapt to new competitive and predatory pressures (Milchunas et al., 1988; Bossdorf et al., 2004) and various ecosystem conditions (Willi et al., 2006) compared to the native range. These selective pressures are highlighted by studies that found significant phenotypic and ecological differences between individuals from native vs invaded ranges (Gallardo et al., 2013; Cerwenka et al., 2014; Courant et al., 2017; Kosmala et al., 2017; Sotka et al., 2018; Dashinov & Uzunova, 2020; Dashinov et al., 2020; Balzani et al., 2021; Phillips & Hagey, 2022; Copilaş-Ciocianu et al., 2023a).
Phenotypic variation is the primary substrate onto which natural selection acts and is therefore of major importance for the establishment of populations in new environments (Fox et al., 2019). Widespread invasive species are likely to exhibit considerable phenotypic variation across their distribution (Evangelista et al., 2019), which facilitates their fitness maintenance both in favourable (opportunistic species) and stressful (robust species) environments (Knop & Reusser, 2012). The high phenotypic diversity of invasive species outside the native range can result from bypassing the bottleneck effect due to multiple introductions (Wattier et al., 2007; Gillis et al., 2009). Phenotypic diversity can also be shaped by the genetic disparities between the source populations in the native area (Hermisson & Wagner, 2004).
Generally, many invasive species are omnivorous, which increases their chances of successful establishment in new environments (Machovsky-Capuska et al., 2016). Usually, the trophic niches of non-native species are broader than those of native species (Feiner et al., 2013; Šidagyte et al., 2017a). Moreover, invasive species can exhibit significant trophic niche and morphological variability across geographic ranges and populations (Jourdan et al., 2019; Copilaş-Ciocianu et al., 2023a). However, the extent to which the evolutionary history in the native range and environmental plasticity can influence the phenotypic differentiation of invasive populations remain poorly investigated.
Functional morphology often reflects an organism’s ecological niche since phenotypes interact with the environment primarily via external morphology (Bock & von Wahlert, 1965; Valen, 1965; Evangelista et al., 2019). As such, functional morphology shapes a species’ spatial distribution and its role in the ecosystem (Ferry-Graham et al., 2002; Dehling et al., 2016), especially from a trophic perspective (Ferry-Graham et al., 2002; Pigot et al., 2020; Copilaş-Ciocianu et al., 2021). Some non-native species undergo morphological and associated dietary changes while colonising new environments (Klepaker, 1993; Adachi et al., 2012; Evangelista et al., 2019). However, the significance of environmental factors in shaping the phenotypic variability of invasive species is still poorly understood (Arbačiauskas et al., 2013).
The Ponto-Caspian region consists of the Black, the Caspian and the Azov Seas and their adjacent lagoons and river deltas (Jażdżewski, 1980). The dynamic geological history and long isolation of the basin promoted diversification and high endemism of various groups of Ponto-Caspian fauna, including crustaceans, molluscs and fish (Cristescu & Hebert, 2005; Griffiths, 2006; Neilson & Stepien, 2009; Wesselingh et al., 2019). Many of them display high phenotypic and environmental plasticity in newly colonised environments (Kostrzewa & Grabowski, 2003; Grabowska et al., 2009; Cerwenka et al., 2014; Copilaş-Ciocianu & Sidorov, 2022). Especially diverse and widely distributed group of Ponto-Caspian fauna are amphipod crustaceans (Väinölä et al., 2008; Copilaş-Ciocianu et al., 2020; Copilaş-Ciocianu et al., 2023b). Among them, particularly widespread are gammarids for which the Ponto-Caspian region constitutes a biodiversity hotspot (Väinölä et al., 2008; Rewicz et al., 2016; Copilaş-Ciocianu & Sidorov, 2022). Almost 40% of these species are invasive and rapidly colonised freshwater ecosystems in Europe (Jażdżewski, 1980; Bij de Vaate et al., 2002; Copilaş-Ciocianu et al., 2023b). Their invasive success is attributed to many biological traits, including diet plasticity, accompanied by a higher predatory ability (Van der Velde et al., 2000; Bącela-Spychalska & Van Der Velde, 2013; Dehedin et al., 2013). However, the morphological variation of traits responsible for feeding across populations from different environments and invasive history is poorly studied.
A good model species for such comparisons is Dikerogammarus villosus (Sowinsky, 1894). It is an invasive amphipod of Ponto-Caspian origin which has broadly spread in Europe (Grabowski et al., 2007; Rewicz et al., 2014; Copilaş-Ciocianu et al., 2023b). Phylogeographic analyses uncovered four genetically distinct native populations along the northwest shore of the Black Sea: the Dnieper Delta, the Dniester Delta, the Danube Delta and the Durungol liman (Rewicz et al., 2015b). Two of these genetically distinct lineages i.e., the Western (the Danube origin) and the Eastern (the Dnieper origin) colonised many European lentic and lotic waters (Rewicz et al., 2015a, b, 2017). The wide distribution of this species in Europe in various lentic and lotic environments could influence its morphological variability, similar to patterns observed in certain fish species (Dürrani et al., 2023; Záhorská et al., 2023). Moreover, this species also experienced one of the strongest climatic niche expansions in the invaded range among invasive Ponto-Caspian amphipods (Šidagytė‐Copilas & Copilaş‐Ciocianu, 2024). Dikerogammarus villosus is described as a crawler ecomorph (Copilaş-Ciocianu & Sidorov, 2022). Amphipods of this ecomorph generally have a slender body and long appendages and hide in coarse substrates such as gravel and stones (Copilaş-Ciocianu & Sidorov, 2022). Dikerogammarus villosus is an omnivorous species demonstrating a broad range of feeding habits (Platvoet et al., 2009; Worischka et al., 2018), which is confirmed by behavioural experiments (Pellan et al., 2015), stable isotopes studies (van Riel et al., 2006; Hellmann et al., 2015) and morphological comparisons of mouthparts (Mayer et al., 2008, 2009; Platvoet et al., 2009; Pellan et al., 2015; Richter et al., 2018). However, some differences in diet and trophic position were observed between certain populations of this species in the River Elbe and the River Rhine (Hellmann et al., 2015), suggesting that some morphological variation might be expected among populations. A recent study by Copilaş-Ciocianu et al. (2023a) has indeed shown that the diet and associated morphological traits of this species differ between the native range in the Black Sea and the invaded range in the Baltic Sea. However, it remains unknown to what extent the environment can influence phenotypic variability and if this variability differs among the two invading lineages. Examining this variability is essential due to its potential to reflect dietary plasticity — a key factor in the invasion process.
Therefore, the goal of our study was to test at the continental scale the effect of environment (brackish waters, freshwater lakes and freshwater river sections), intraspecific lineage (Western, Dniester and Eastern) and range (native and invaded) in shaping the variability of functional morphological traits that directly (gnathopods, mouthparts, stomach) or indirectly (antennae, walking legs) reflect the diet of Dikerogammarus villosus. Given its broad geographical occurrence in different types of waterbodies (freshwater river sections, brackish waters and freshwater lakes) and its trophic plasticity, we hypothesise that D. villosus exhibits a considerable amount of functional morphological variation. We further hypothesise that due to greater environmental heterogeneity in the invaded range, D. villosus experiences a significant morphospace expansion outside the native range. Understanding this variation is important for better comprehension of the invasive potential of this species.
Materials and methods
Sampling and laboratory procedures
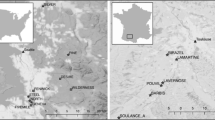
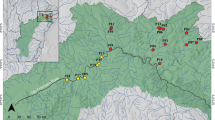
The examined material consisted of male specimens of Dikerogammarus villosus collected from 35 sampling points across three different environments i.e., freshwater river sections, freshwater lakes and brackish waters, in both native and invaded ranges in Europe (as illustrated in Fig. 1 and detailed in Supplementary Table 1). We considered all of the native sampling sites as belonging to the brackish water category as they are located either in brackish coastal lagoons or in deltaic regions, which are regularly subjected to saline water intrusions. Some environmental factors may be unique to each environment type but multiple may be shared. Therefore, our division of environments is based mainly on salinity (brackish waters vs freshwater river sections and lakes) and water current (brackish waters and rivers vs lakes). These two main environmental factors were used in the discussion of our results. Based on Rewicz et al. (2015b), we divided our dataset into Western, Eastern and Dniester intraspecific genetic lineages. Specimens were collected at a depth of up to 0.5 m through “kick-sampling” with a benthic hand-net with a mesh size of 0.5 mm according to established protocols of Jażdżewski et al. (2002) and Grabowski et al. (2006). The amphipods were preserved in 96% ethanol and then identified in the laboratory to the species level based on the literature (Mordukhay-Boltovskoy, 1964; Eggers & Martens, 2001).
From most localities, 10 mature, well-preserved individuals, without visible damage to the body and appendages, were chosen for the dissection. In the case of five sampling points, a smaller number of specimens (six specimens from each of two localities and nine individuals from each of three localities) was used, depending on material availability. In total, we used 339 individuals (9.7 ind./locality on average) for the dissection. Only male specimens were chosen as we wanted to exclude sexual dimorphism as a confounding factor (Conlan, 1991). Before the dissection, the cuticle was softened by immersing the specimens overnight in 1.5% lactic acid solution as in Zhao et al. (2021) and subsequently stored for a few hours in 1:3 glycerol-ethanol mix as in Copilaş-Ciocianu et al. (2021).
For assessing functional morphological differentiation, we chose 29 traits involved in sensory functions (both antennae), food processing and digestion (stomach, mandibles, maxillipeds), food capturing and handling (the first pair of gnathopods) and locomotion (the third and the seventh pair of pereiopods, the first pair of pleopods and the third pair of uropods) (see Supplementary Table 2). All traits were chosen according to Copilaş-Ciocianu et al. (2021). For comparative purposes, always the right body side was dissected as in Copilaş-Ciocianu et al. (2021). The left side was used for the dissection only when the appendages on the right body side were damaged. Always the right-side mandibles were dissected to take into account their asymmetry (Mayer et al., 2012). The dissections were conducted under the stereomicroscope using needles, fine tweezers and microsurgical scissors according to Copilaş-Ciocianu et al. (2021) and Zhao et al. (2021). Dissected appendages were mounted on microscope slides in glycerol and photographed under a Nikon SMZ1000 stereomicroscope with a Pixelink M15C-CYL camera. Afterwards, the measurements were conducted based on photographs in Digimizer 4 software. The landmarks were chosen according to Fišer et al. (2009) and Copilaş-Ciocianu et al. (2021).
Statistical analysis
Measurements (except the gnathopod palmar angle) were regressed against body length to remove the effect of body size. In the subsequent analyses, we used regression residuals. Specimens that showed outlying values (mean ± 2 × SD) were excluded from further analysis. Morphological traits were either analysed altogether or separated into four functional groups of traits i.e., sensory functions (antennae, six measurements), food processing and digestion (mouthparts and stomach, four measurements), food capturing and handling (gnathopods, seven measurements) and locomotion (pereiopods, pleopods and uropods, 10 measurements). Body and head lengths can be a proxy of multiple ecological functions (Allen et al., 2006), therefore, were not assigned to any functional group and analysed only in the set of all traits altogether. A Permutational Multivariate Analysis of Variance (PERMANOVA) with 999 permutations was used to test for morphological differences (either all traits or split among the four functional groups) between three grouping factors i.e., geographic range (two levels: native and invaded ranges), lineage (three levels: Western, Dniester and Eastern) and environment (three levels: rivers (freshwater sections), lakes (freshwater), brackish waters). Populations were assigned an invasion range based on Copilaş-Ciocianu et al. (2023a), and lineage assignment followed Rewicz et al. (2017). Both the effects of factors as well as all the possible interactions between them were tested. However, because the native range in our study contains only one type of environment (brackish waters, see above), the range: environment interaction as well as the full lineage: environment: range interaction could not be tested. To avoid pseudoreplication, due to measurements of multiple specimens per locality, the population factor was included in the analysis as strata during the permutations. The Euclidean distance metric was used to measure dissimilarity between data points. Pairwise comparisons were conducted under the Bonferroni correction. All PERMANOVA tests were performed in R 4.3.0 (R Core Team, 2023) using adonis2 function of the package vegan and pairwise.adonis of the package pairwiseAdonis for the post hoc analysis between levels of the significant factors (Martinez Arbizu, 2020). To visually explore the patterns of differentiation, Principal Component Analysis (PCA) using a Pearson Correlation matrix was performed in PAST 4 (Hammer et al., 2001).
To estimate the magnitude and patterns of morphological differentiation between lineages, environments and geographic ranges, the n-dimensional hypervolume approach was applied to the first two PCA dimensions (PC1, PC2) as in Copilaş-Ciocianu et al. (2023b). Due to the sake of comparability among functional trait groups only the first two PCA dimensions were included. Hypervolumes for native and invaded ranges were constructed by pooling all individuals from the Western and Eastern Lineages as well as separately for each of the two lineages. Individuals from the Dniester Lineage were excluded from the range hypervolume analysis as this lineage is currently not known to occur outside its native range. Additionally, we also tested which of the environments had the greatest effect on morphospace change in the invaded range compared with the native. For this, we conducted a pairwise hypervolume comparison among the native brackish environment with each of the three environments in the invaded range separately (i.e., native brackish waters vs invaded freshwater river sections; native brackish waters vs invaded freshwater lakes and native brackish waters vs invaded brackish waters) by accounting for each of the two invading lineages separately (the Western and the Eastern). All hypervolume pairs were constructed using the hypervolume v. 3.1.0. R package (Blonder et al., 2014, 2018, 2023). For each hypervolume pair, we calculated total and unique volumes, distances between centroids and the Jaccard index. Furthermore, we estimated morphological change dynamics (i.e., expansion, contraction and shift) among geographic ranges with the R package BAT v.2.9.2. (Cardoso et al., 2015). The change was assessed with the βtotal diversity index (= 1 − a value of Jaccard similarity), ranging from 0 for fully overlapping morphospaces, and 1 for completely non-overlapping morphospaces. Subsequently, this index was decomposed into the βreplacement index, indicating morphospace shift, and the βrichness index, indicating morphospace contraction or expansion (Carvalho & Cardoso, 2020). We highlight that this terminology should not be confounded with the sequence of the invasion process. Therefore, it should be only considered as a change of morphospace between geographic ranges and not as changes with time.
Results
The PERMANOVA test showed that environment type has the most significant effect on the total (all traits combined) morphological differentiation (F = 16.20, P = 0.001), followed by lineage (F = 5.35, P = 0.003) and range (F = 4.17, P = 0.023) (Table 1). Pairwise comparisons (see Supplementary File 3) indicate significant differences between the Western and the Eastern Lineages (P = 0.002) and between the Eastern and the Dniester Lineages (P = 0.020). Regarding environments, significant differences were observed between brackish waters and rivers/lakes (P < 0.001/P = 0.002, respectively) but not between lakes and rivers. Significant differences between the Western and the Eastern Lineages were observed in lakes (P = 0.025) and in brackish waters between the Eastern and the Dniester Lineages (P < 0.001). Within the Western and Eastern Lineages, brackish waters differ compared to the rivers (both P < 0.001) and lakes (P = 0.017, P = 0.005, respectively).
Sensory traits (antennae) differ between environments (F = 4.21, P = 0.003) and in the interaction between environments and lineages (F = 7.09, P = 0.001) (Table 1). Significant differences were detected between lakes and rivers (P = 0.019) as well as between lakes and brackish waters (P = 0.001). The Western and the Eastern Lineages differ in lakes (P < 0.001). Within the Eastern Lineage lakes differ significantly from brackish waters and rivers (both P < 0.001) (Supplementary File 3).
Food processing and digestion traits (mouthparts and stomach) differ significantly between environments (F = 14.84, P = 0.001), lineages (F = 2.83, P = 0.046) and in the interaction between lineages and environments (F = 8.70, P = 0.001) (Table 1). All environments differ from each other (P < 0.05), while the differences between lineages were observed between the Dniester and two other lineages (P = 0.020, P = 0.036 for comparison with the Western and the Eastern, respectively). Within the Eastern Lineage, all environments differ from each other (P < 0.05), while for the Western Lineage, brackish waters significantly differ from lakes and rivers (P = 0.013, P = 0.034, respectively). All the lineages differ from each other in brackish waters (P < 0.05). Additionally, the Western and the Eastern Lineages differ in lakes (P = 0.010) (Supplementary File 3).
Food capturing and handling traits (gnathopods) differ significantly between environments (F = 22.00, P = 0.001) and lineages (F = 4.45, P = 0.011) (Table 1). These traits differ between the Western and the Eastern Lineages (P = 0.008) and between populations from brackish waters and other environments (P < 0.05). Within the Eastern Lineage, populations from brackish waters significantly differ from other environments (P < 0.001 and P = 0.006 for comparisons with rivers and lakes, respectively). Within the Western Lineage, populations from brackish waters also differ from rivers and lakes (P < 0.001, P = 0.019, respectively). Brackish populations differ between the Eastern Lineage and two other lineages (P < 0.05) (Supplementary File 3).
Locomotion traits (pereiopods, pleopods and uropods) significantly differ between native and invaded ranges (F = 8.37, P = 0.001) and between lineages (F = 5.33, P = 0.002) (Table 1). Pairwise comparisons for lineages showed a significant difference between the Western and two other lineages (P < 0.05). Within the Eastern lineage, significant differences were observed between lakes and rivers (P = 0.001) as well as between lakes and brackish waters (P = 0.002). Populations from brackish waters differ between the Dniester and the Eastern Lineages (P = 0.042) as well as between river populations from the Western and the Eastern Lineages (P < 0.001) (Supplementary File 3).
In the PCA analysis, the first two axes explain 47.8% of the morphological variation. The first axis (39.8% of variation explained) reflects the length of pereiopods, mandible palps and peduncles of antennae, while the second axis (8.0% of variation explained) reflects the molar surface, palmar angle, and length of stomach, head, gnathopod palm, spines and setae of gnathopods as well as maxilliped palps (Fig. 2). Overall, populations from brackish waters are characterised by a tendency towards a narrower palmar angle, smaller molar surface and stomach length and have an increased body size and head length as well as palmar spines (Fig. 2b). Morphological variation increases in the invaded range, with populations being characterised by generally longer antennae and pereiopods compared to native populations (Fig. 2c).
PCAs for separate groups of traits (see Fig. 3a) increased the percentage of variation explained. PCA for sensory traits (86.2% of variation explained) indicates that populations from rivers and brackish waters have generally shorter antennae (Fig. 3b). PCA for food processing and digestion traits (69.7% of variation explained) indicates a trend towards decreasing stomach length and molar surface in populations from brackish waters (Fig. 3c) and from the Western Lineage (Fig. 3d). PCA for food capturing traits (66.8% of variation explained) indicates that populations from brackish waters have generally smaller palmar angles and longer palms, spines and setae of gnathopods relative to freshwater populations (Fig. 3e). A trend towards increasing gnathopod size was observed in the Western Lineage (Fig. 3f). PCA for locomotion traits (67% of variation explained) indicated a trend towards decreasing the length of the 7th pair of pereiopods in the native area (Fig. 3g) and for the Dniester Lineage (Fig. 3h).
PCA scatterplots of morphological differentiation among populations of D. villosus across environment types, ranges and lineages concerning functional groups of traits. Scheme highlighting the location and composition of functional groups (a). Only statistically significant combinations from PERMANOVA analysis for each group of traits are illustrated (b–h). Abbreviations of the traits according to Supplementary Table 2
Hypervolumes indicate that the highest morphospace overlap is found among the Western and the Eastern Lineages when ranges are disregarded (Jaccard = 0.74) (Table 2, Fig. 4d). Considering only ranges, the amount of overlap decreases (Jaccard = 0.52) (Table 2, Fig. 4a). When both lineage and range are factored in, the amount of overlap decreases even more, with a moderate overlap among ranges within the Western Lineage (Jaccard = 0.48) (Fig. 4b), and a small overlap among ranges within the Eastern Lineage (Jaccard = 0.27) (Fig. 4c). Morphospace overlap among environments mirrors the PERMANOVA and PCA results, with the lowest overlap being observed among brackish and river/lake populations (Jaccard = 0.51 and 0.41, respectively) and the highest between rivers and lakes (Jaccard = 0.64) (Table 2, Fig. 4e).
Analysis of niche change dynamics reveals a morphospace expansion in the invaded range when lineages are pooled together (native volume = 67.36; invaded volume = 109.43) with the βrichness explaining 75% of the total (βtotal) differentiation (Table 2). When lineages are considered, niche change dynamics among ranges become more refined and lineage-specific, with the Western Lineage being characterised more by a shift accompanied by an expansion (native volume = 81.25; invaded volume = 103.20; βreplacement = 66% of βtotal) while the Eastern Lineage underwent a significant overall morphospace expansion (native volume = 36.94; invaded volume = 129.98; βrichness = 96% of βtotal).
Morphospace change dynamics among ranges differ according to the environment. The Western Lineage can be characterised by an expansion in brackish waters (native volume = 80.69; invaded volume = 137.21; βrichness = 76% of βtotal), a shift with a slight contraction in lakes (native volume = 80.69; invaded volume = 67.07; βreplacement = 82% of βtotal) and a shift in rivers (native volume = 80.69; invaded volume = 82.99; βreplacement = 97% of βtotal) (Table 2, Fig. 5a–c). While the Eastern Lineage can be characterised by an expansion in all environments in the invaded range i.e., in brackish waters (native volume = 37.07; invaded volume = 90.81; βrichness = 89% of βtotal), in lakes (native volume = 37.07; invaded volume = 81.98; βrichness = 75% of βtotal) and in rivers (native volume = 37.07; invaded volume = 60.78; βrichness = 69% of βtotal) (Table 2, Fig. 5d–f).
Discussion
Our results reveal that Dikerogammarus villosus exhibits a substantial amount of morphological variability across Europe. The main driver for this variation seems to be environment type as we uncovered that brackish water populations differ the most from populations inhabiting rivers and lakes. Differentiation between intraspecific lineages and among geographic ranges (native and invaded) is significant, but not as strong. Furthermore, we found that the two invading lineages (Western and Eastern) exhibit unique patterns of increasing morphological disparity in the invaded range, especially in brackish waters. Below we discuss the implications of these findings and their significance for the ongoing invasion of this species.
Individuals from brackish waters are characterised by longer setae on gnathopods propodi. This setation plays a role in filtering food particles, grooming and transferring to the mouthparts (Platvoet et al., 2006; Mayer et al., 2012). We also observed that the specimens from brackish waters have longer palms and narrower palmar angles. This increases the size of the opening between the dactylus and propodus, thus favouring the capture and handling of larger prey (Loxton & Nicholls, 1979; Fišer et al. (2019); Premate et al., 2021). These observations together with generally longer gnathopods of amphipods in brackish waters suggest that individuals from these populations can handle larger prey items, and as such could be more predatory.
The possibly higher predatory nature of brackish populations of D. villosus can be also evidenced by the modification of food processing and digestion traits. Plant material is less nutritious and energy efficient (Pellan et al., 2015). Therefore, herbivorous organisms need to consume a high amount of plant material to compensate for their energetic needs. Consequently, herbivorous amphipods have a larger stomach and a broader molar surface than carnivorous species (Coleman, 1991; Mayer et al. (2015), Watling (1993); Copilaş-Ciocianu et al., 2021). Indeed, we observed that D. villosus specimens from brackish waters have shorter stomachs and smaller molar surfaces than specimens from other populations, suggesting a possibly higher tendency towards carnivory (higher specialisation). This again indicates that brackish waters individuals may be more predatory than those in freshwater environments.
Amphipods detect prey using their antennae, hence relatively long antennae are thought to be more common in predatory species or populations (Copilaş-Ciocianu et al., 2021). We observed longer antennae among lake populations compared to brackish environments, which stands in contrast to the suggested higher carnivory of brackish populations. However, we can assume that their length is related to environmental conditions. Studies on hermit crabs show that chemical cues detection can be disturbed by water pH (De la Haye et al., 2012). In the case of amphipods, it is known that the environment can have an impact on the morphology of antennae (Jones & Culver, 1989; Delić et al., 2016). Indeed, we can speculate that lower pH in eutrophic lakes favours longer antennae for more efficient chemical detection. Furthermore, the length of the antennae may be also determined by the water current (Delić et al., 2016), and therefore, we may expect that specimens inhabiting lakes need longer antennae to orientate efficiently in a habitat with lower water currents compared with rivers and river mouths. Moreover, antennae are also responsible for filter feeding (Platvoet et al., 2006; Fišer et al., 2009), thus, standing in congruence with our observations. Namely, our previous conclusions claiming more herbivory and detritus feeding of freshwater populations may be an explanation for the observed trend. However, these observations need to be further studied and completed with experimental testing.
Considering the above, we can generally assume that brackish populations are more carnivorous than freshwater populations. Indeed, stable isotope analysis on the closely related Pontogammarus robustoides showed a higher trophic position (reflecting higher predation) of populations from brackish waters than freshwater environments (Arbačiauskas et al., 2013). It has been hypothesised that the higher phosphorus and lower nitrogen contents in brackish waters promote predation and faster growth rates (Arbačiauskas et al., 2013). Our results suggest that the putatively increased carnivory of brackish populations of D. villosus may cause a more severe impact on macrobenthic communities and more rapidly spread in coastal areas of the Baltic Sea (Šidagytė et al., 2017b; Copilaş-Ciocianu & Šidagytė-Copilaş, 2022).
We also observed morphological differences between ranges (i.e., native vs invaded). Specimens in the native range have a slightly narrower palmar angle of gnathopods of the 1st pair compared to the invaded range. It suggests more predatory habits of D. villosus in the native range and higher omnivory in the invaded range. Indeed, omnivorous habits are an important trait promoting the successful invasion of this species (Van der Velde et al., 2000; van Riel et al., 2006; Platvoet et al., 2009). Our findings are supported by a recent study that indicated a niche contraction in the invaded range with a shift towards decreased carnivory (Copilaş-Ciocianu et al., 2023a). However, the differences observed in our study are driven mainly by the environment. For instance, the palmar angle of gnathopods of the 1st pair differs between individuals of the Eastern Lineage from brackish waters in native and invaded ranges. A narrower palmar angle in the case of amphipods from Baltic populations (invaded range of the Eastern Lineage) underline their higher level of predatory and possible threat to the macrofauna of the Eastern coast of the Baltic Sea.
At the lineage level, we observed a significant differentiation with respect to the locomotor apparatus and food processing traits. Individuals from the Western populations have longer pereiopods, compared to those from the Eastern populations. The same can be observed for individuals from the invaded range in comparison to the native range. These appendages are responsible for locomotion, and their length positively influences locomotion speed (Kralj-Fišer et al., 2020; Boudrias (2002), Dahl (1978)). An enhancement of the spreading speed in the invaded range was observed for instance in cane toads (Kosmala et al., 2017). It can be assumed that predatory specimens might have longer pereiopods (Copilaş-Ciocianu et al., 2021), suggesting a higher predatory ability of D. villosus individuals from the Western Lineage and invaded range. The higher predatory ability of the Western Lineage can be also evidenced by bigger gnathopods. In contrast, we show that the populations from the Eastern Lineage have longer stomachs and broader molar surfaces, which might reflect a higher amount of plant material in their diet. We also find that the morphology of the Dniester Lineage, which is restricted only to the native Dniester lagoon, overlaps significantly with the Western and Eastern Lineages. This indicates that it has an intermediate morphology, which reflects its genetically intermediate position between the Western and Eastern Lineages (Rewicz et al., 2015b).
Each of the two invasive lineages displays a unique pattern of morphological change in the invaded range compared with the native area. We observed a morphospace shift in the invaded range within the Western Lineage and a morphospace expansion within the Eastern Lineage. Although the morphospace of the Eastern Lineage in the native range is smaller than that of the Western Lineage, it is larger in the invaded range. However, the factors behind this disparity could be multiple. One reason could be due to the possibly higher heterogeneity of the invaded environments in Eastern Europe, where there are fewer artificial channels and waters are less modified (Bij de Vaate et al., 2002). The Eastern Lineage also experienced a significant morphospace expansion in all three environment types in the invaded range which may suggest an intrinsically higher developmental plasticity than the Western Lineage. Regardless, one could assume that the more variable Eastern Lineage may be more successful in invading new habitats. Although fewer studies were done on the Eastern Lineage, they show a progressive expansion of D. villosus in the coastal areas of the Baltic Sea (Šidagytė et al., 2017b; Copilaş-Ciocianu & Šidagytė-Copilaş, 2022) but also in freshwaters of the Masurian Lakeland (Podwysocki et al., 2024).
Our results constitute an important contribution to the study of morphological variability and plasticity of invasive aquatic species. The high morphological disparity observed between populations of D. villosus from different environments, as well as among ranges and evolutionary lineages underlines the importance of incorporating environmental and evolutionary factors across a wide geographical area and not limiting these comparisons among the native and invaded ranges. Although the environment is the main driver of the observed variance, the differentiation among lineages and ranges suggests differences in plasticity between lineages. In particular, variation of traits responsible for food processing and digestion, can be an important driver of trophic niche expansion or shift in the newly colonised environments. However, experimental studies are critical for gaining a better comprehension of how morphological plasticity is reflected ecologically. Furthermore, experimental findings would also need to be validated with a complementary analysis of the diet (stable isotopes and gut content) of wild populations. Possible dietary differences between populations could also result from the chemical composition and ultrastructure of mouthparts, warranting further research in this direction (Mekhanikova et al., 2012).
Conclusion
Our study revealed that Dikerogammarus villosus, one of the most prominent invaders in Europe, exhibits a remarkable amount of morphological variability at the continental scale, especially in functional traits related to diet. Although the environment is the main driver of morphological divergence, intraspecific lineages and invasion history also play an important role. Moreover, the two invading lineages exhibit unique dynamics of morphological change in the invaded range relative to the native range, suggesting a lineage-specific invasion potential. The high morphological variability suggests a high level of plasticity, which likely reflects its high genetic diversity in the invaded range. This indicates a fast adaptive potential that promotes expansion and successful establishment in new habitats.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adachi, T., A. Ishikawa, S. Mori, W. Makino, M. Kume, M. Kawata & J. Kitano, 2012. Shifts in morphology and diet of non-native sticklebacks introduced into Japanese crater lakes. Ecology and Evolution 2: 1083–1098.
Allen, C. R., A. S. Garmestani, T. D. Havlicek, P. A. Marquet, G. D. Peterson, C. Restrepo, C. A. Stow & B. E. Weeks, 2006. Patterns in body mass distributions: sifting among alternative hypotheses. Ecology Letters 9: 630–643. https://doi.org/10.1111/j.1461-0248.2006.00902.x.
Arbačiauskas, K., J. Lesutienė & Z. R. Gasiūnaite, 2013. Feeding strategies and elemental composition in Ponto-Caspian peracaridans from contrasting environments: can stoichiometric plasticity promote invasion success? Freshwater Biology 58: 1052–1068.
Atwood, J. & L. Meyerson, 2011. Beyond EICA: understanding post-establishment evolution requires a broader evaluation of potential selection pressures. NeoBiota 10: 7–25.
Bącela-Spychalska, K. & G. Van Der Velde, 2013. There is more than one “killer shrimp”: trophic positions and predatory abilities of invasive amphipods of Ponto-Caspian origin. Freshwater Biology 58: 730–741.
Balzani, P., S. Vizzini, F. Frizzi, A. Masoni, J. P. Lessard, C. Bernasconi, A. Francoeur, J. Ibarra-Isassi, F. Brassard, D. Cherix & G. Santini, 2021. Plasticity in the trophic niche of an invasive ant explains establishment success and long-term coexistence. Oikos 130: 691–696.
Bij de Vaate, A., K. Jażdzewski, H. A. M. Ketelaars, S. Gollasch & G. Van der Velde, 2002. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 59: 1159–1174.
Blonder, B., C. Lamanna, C. Violle & B. J. Enquist, 2014. The n-dimensional hypervolume. Global Ecology and Biogeography 23: 595–609.
Blonder, B., C. B. Morrow, B. Maitner, D. J. Harris, C. Lamanna, C. Violle, B. J. Enquist & A. J. Kerkhoff, 2018. New approaches for delineating n-dimensional hypervolumes. Methods in Ecology and Evolution 9: 305–319.
Blonder, B., C. B. Morrow, S. Brown, G. Butruille, D. Chen, A. Laini, & D. J. Harris, 2023. Package ‘ hypervolume .’ 92.
Bock, W. J. & G. von Wahlert, 1965. Adaptation and the form-function complex. International Journal of Ogranic Evolution 19: 1965.
Bossdorf, O., D. Prati, H. Auge & B. Schmid, 2004. Reduced competitive ability in an invasive plant. Ecology Letters 7: 346–353.
Boudrias, M. A., 2002. Are pleopods just “more legs”? The functional morphology of swimming limbs in Eurythenes gryllus (Amphipoda). Journal of Crustacean Biology 22: 581–594.
Cardoso, P., F. Rigal & J. C. Carvalho, 2015. BAT—biodiversity assessment tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods in Ecology and Evolution 6: 232–236.
Carvalho, J. C. & P. Cardoso, 2020. Decomposing the causes for niche differentiation between species using hypervolumes. Frontiers in Ecology and Evolution 8: 1–7.
Cerwenka, A. F., P. Alibert, J. Brandner, J. Geist & U. K. Schliewen, 2014. Phenotypic differentiation of Ponto-Caspian gobies during a contemporary invasion of the upper Danube River. Hydrobiologia 721: 269–284.
Coleman, C. O., 1991. Comparative fore-gut morphology of Antarctic Amphipoda (Crustacea) adapted to different food sources. Hydrobiologia 223: 1–9.
Conlan, K. E., 1991. Precopulatory mating behavior and sexual dimorphism in the amphipod Crustacea. Hydrobiologia 223: 255–282.
Copilaş-Ciocianu, D. & E. Šidagytė-Copilaş, 2022. A substantial range expansion of alien Ponto-Caspian amphipods along the eastern Baltic Sea coast. Oceanologia 64: 227–232.
Copilaş-Ciocianu, D. & D. Sidorov, 2022. Taxonomic, ecological and morphological diversity of Ponto-Caspian gammaroidean amphipods: a review. Organisms Diversity and Evolution 22: 285–315. https://doi.org/10.1007/s13127-021-00536-6.
Copilaş-Ciocianu, D., Š Borko & C. Fišer, 2020. The late blooming amphipods: global change promoted post-Jurassic ecological radiation despite Palaeozoic origin. Molecular Phylogenetics and Evolution 143: 106664.
Copilaş-Ciocianu, D., B. V. Boros & E. Šidagytė-Copilas, 2021. Morphology mirrors trophic niche in a freshwater amphipod community. Freshwater Biology 66: 1968–1979.
Copilaş-Ciocianu, D., A. Garbaras & E. Šidagytė-Copilas, 2023a. Invasion is accompanied by dietary contraction in Ponto-Caspian amphipods. bioRxiv. https://doi.org/10.1101/2023.08.08.552405.
Copilaş-Ciocianu, D., D. Sidorov & E. Šidagytė-Copilas, 2023b. Global distribution and diversity of alien Ponto-Caspian amphipods. Biological Invasions 25: 179–195. https://doi.org/10.1007/s10530-022-02908-1.
Courant, J., S. Vogt, R. Marques, J. Measey, J. Secondi, R. Rebelo, A. De Villiers, F. Ihlow, C. De Busschere, T. Backeljau, D. Rödder & A. Herrel, 2017. Are invasive populations characterized by a broader diet than native populations? PeerJ 5: e3250.
Cristescu, M. E. A. & P. D. N. Hebert, 2005. The “Crustacean Seas”—an evolutionary perspective on the Ponto-Caspian peracarids. Canadian Journal of Fisheries and Aquatic Sciences 62: 505–517.
Dahl, E., 1978. The amphipod functional model and its bearing upon systematics and phylogeny. Zoologica Scripta 6: 221–228. https://doi.org/10.1111/j.1463-6409.1978.tb00773.x.
Dashinov, D. & E. Uzunova, 2020. Diet and feeding strategies of round goby, Neogobius melanostomus (Pallas, 1814) from the invasion front in the Danube River tributaries (Bulgaria): ontogenetic shift and seasonal variation. Limnologica 83: 125796. https://doi.org/10.1016/j.limno.2020.125796.
Dashinov, D., P. Czerniejewski, S. Balshine, C. Synyshyn, E. Tasheva-Terzieva, T. Stefanov, P. Ivanova, N. Mandrak & E. Uzunova, 2020. Variation in external morphology between the native and invasive populations of the round goby, Neogobius melanostomus (Actinopterygii: Gobiidae). Zoomorphology 139: 361–371. https://doi.org/10.1007/s00435-020-00480-7.
De la Haye, K. L., J. I. Spicer, S. Widdicombe & M. Briffa, 2012. Reduced pH sea water disrupts chemo-responsive behaviour in an intertidal crustacean. Journal of Experimental Marine Biology and Ecology 412: 134–140. https://doi.org/10.1016/j.jembe.2011.11.013.
Dehedin, A., C. Maazouzi, S. Puijalon, P. Marmonier & C. Piscart, 2013. The combined effects of water level reduction and an increase in ammonia concentration on organic matter processing by key freshwater shredders in alluvial wetlands. Global Change Biology 19: 763–774.
Dehling, D. M., P. Jordano, H. M. Schaefer, K. Böhning-Gaese & M. Schleuning, 2016. Morphology predicts species’ functional roles and their degree of specialization in plant–frugivore interactions. Proceedings of the Royal Society b: Biological Sciences 283: 20152444.
Delić, T., P. Trontelj, V. Zakšek & C. Fišer, 2016. Biotic and abiotic determinants of appendage length evolution in a cave amphipod. Journal of Zoology 299: 42–50.
Dürrani, Ö., T. Ateşşahin, M. Eroğlu & M. Düşükcan, 2023. Morphological variations of an invasive cyprinid fish (Carassius gibelio) in lentic and lotic environments inferred from the body, otolith, and scale shapes. Acta Zoologica 104: 458–472.
Eggers, T. O. & A. Martens, 2001. Bestimmungsschlüssel der Süßwasser-Amphipoda (Crustacea) Deutschlands. A key to the freshwater Amphipoda (Crustacea) of Germany. Lauterbornia 42: 1–68.
Evangelista, C., J. D. Olden, A. Lecerf & J. Cucherousset, 2019. Scale-dependent patterns of intraspecific trait variations in two globally invasive species. Oecologia 189: 1083–1094. https://doi.org/10.1007/s00442-019-04374-4.
Feiner, Z. S., J. A. Rice & D. D. Aday, 2013. Trophic niche of invasive white perch and potential interactions with representative reservoir species. Transactions of the American Fisheries Society 142: 628–641.
Ferry-Graham, L. A., D. I. Bolnick & P. C. Wainwright, 2002. Using functional morphology to examine the ecology and evolution of specialization. Integrative and Comparative Biology 42: 265–277.
Fišer, C., P. Trontelj, R. Luštrik & B. Sket, 2009. Toward a unified taxonomy of Niphargus (Crustacea: Amphipoda): a review of morphological variability. Zootaxa 2061: 1–22.
Fišer, C., T. Delić, R. Luštrik, M. Zagmajster & F. Altermatt, 2019. Niches within a niche: Ecological differentiation of subterranean amphipods across Europe’s interstitial waters. Ecography 42: 1212–1223. https://doi.org/10.1111/ecog.03983.
Fox, R. J., J. M. Donelson, C. Schunter, T. Ravasi & J. D. Galtan-Espitia, 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philosophical Transactions of the Royal Society B 374: 20180174. https://doi.org/10.1098/rstb.2018.0174.
Gallardozu Ermgassen, B. P. S. E. & D. C. Aldridge, 2013. Invasion ratcheting in the zebra mussel (Dreissena polymorpha) and the ability of native and invaded ranges to predict its global distribution. Journal of Biogeography 40: 2274–2284.
Gillis, N. K., L. J. Walters, F. C. Fernandes & E. A. Hoffman, 2009. Higher genetic diversity in introduced than in native populations of the mussel Mytella charruana: evidence of population admixture at introduction sites. Diversity and Distributions 15(5): 784–795. https://doi.org/10.1111/j.1472-4642.2009.00591.x.
Grabowska, J., M. Grabowski & A. Kostecka, 2009. Diet and feeding habits of monkey goby (Neogobius fluviatilis) in a newly invaded area. Biological Invasions 11: 2161–2170.
Grabowski, M., A. Konopacka, K. Jażdżewski & E. Janowska, 2006. Invasions of alien gammarid species and retreat of natives in the Vistula Lagoon (Baltic Sea, Poland). Helgoland Marine Research 60: 90–97.
Grabowski, M., K. Bącela & A. Konopacka, 2007. How to be an invasive gammarid (Amphipoda: Gammaroidea)—comparison of life history traits. Hydrobiologia 590: 75–84.
Griffiths, A., 2006. Pattern and process in the ecological biogeography of European freshwater fish. Journal of Animal Ecology 75: 734–751.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. Past: paleontological statistics software package for education. Palaeontologia Electronica 4: 1–9.
Hellmann, C., S. Worischka, E. Mehler, J. Becker, R. Gergs & C. Winkelmann, 2015. The trophic function of Dikerogammarus villosus (Sowinsky, 1894) in invaded rivers: a case study in the Elbe and Rhine. Aquatic Invasions 10: 385–397.
Hermisson, J. & P. Wagner, 2004. The population genetic theory of hidden variation and genetic robustness. Genetics 168: 2271–2284. https://doi.org/10.1534/genetics.104.029173.
Jażdżewski, K., 1980. Range extensions of some Gammaridean species in European inland waters caused by human activity. Crustaceana 84–107.
Jażdżewski, K., A. Konopacka & M. Grabowski, 2002. Four Ponto-Caspian and one American gammarid species (Crustacea, Amphipoda) recently invading Polish waters. Contributions to Zoology 71: 115–122.
Jones, R. & D. C. Culver, 1989. Evidence for selection on sensory structures in a cave population of Gammarus minus (Amphipoda). Evolution 43: 688–693.
Jourdan, J., K. Piro, A. Weigand & M. Plath, 2019. Small-scale phenotypic differentiation along complex stream gradients in a non-native amphipod. Frontiers in Zoology Frontiers in Zoology 16: 1–20.
Klepaker, T., 1993. Morphological changes in a marine population of threespined stickleback, Gasterosteus aculeatus, recently isolated in fresh water. Canadian Journal of Zoology 71: 1251–1258.
Knop, E. & N. Reusser, 2012. Jack-of-all-trades: phenotypic plasticity facilitates the invasion of an alien slug species. Proceedings of the Royal Society b: Biological Sciences 279: 4668–4676.
Kosmala, G., K. Christian, G. Brown & R. Shine, 2017. Locomotor performance of cane toads differs between native-range and invasive populations. Royal Society Open Science 4: 170517. https://doi.org/10.1098/rsos.170517.
Kostrzewa, J. & M. Grabowski, 2003. Opportunistic feeding strategy as a factor promoting the expansion of racer goby (Neogobius gymnotrachelus Kessler, 1857) in the Vistula basin. Lauterbornia 48: 91–100.
Kralj-Fišer, S., E. Premate, D. Copilaş-Ciocianu, T. Volk, Ž Fišer, G. Balázs, G. Herczeg, T. Delić & C. Fišer, 2020. The interplay between habitat use, morphology and locomotion in subterranean crustaceans of the genus Niphargus. Zoology 139: 125742. https://doi.org/10.1016/j.zool.2020.125742.
Loxton, R. G. & I. Nicholls, 1979. The functional morphology of the praying mantis forelimb (Dictyoptera: Mantodea). Zoological Journal of the Linnean Society 66: 185–203.
Machovsky-Capuska, G. E., A. M. Senior, S. J. Simpson & D. Raubenheimer, 2016. The multidimensional nutritional niche. Trends in Ecology and Evolution 31: 355–365. https://doi.org/10.1016/j.tree.2016.02.009.
Martinez Arbizu, P., 2020. pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4
Mayer, G., G. Maier, A. Maas & D. Waloszek, 2008. Mouthparts of the Ponto-Caspian invader Dikerogammarus villosus (Amphipoda: Pontogammaridae). Journal of Crustacean Biology 28: 1–15.
Mayer, G., G. Maier, A. Maas & D. Waloszek, 2009. Mouthpart morphology of Gammarus roeselli compared to a successful invader, Dikerogammarus villosus (Amphipoda). Journal of Crustacean Biology 29: 161–174.
Mayer, G., A. Maas & D. Waloszek, 2012. Mouthpart morphology of three sympatric native and nonnative gammaridean species: Gammarus pulex, G. fossarum, and Echinogammarus berilloni (Crustacea: Amphipoda). International Journal of Zoology 2012: 1–23.
Mayer, G., A. Maas & D. Waloszek, 2015. Mouthpart morphology of Synurella ambulans (F. Müller, 1846). Spixiana 38: 219–229.
Mekhanikova, I. V., D. S. Andreev, O. Y. Belozerova, Y. L. Mikhlin, S. V. Lipko, I. V. Klimenkov, V. V. Akimov, V. F. Kargin, Y. V. Mazurova, V. L. Tauson & Y. V. Likhoshway, 2012. Specific features of mandible structure and elemental composition in the polyphagous amphipod Acanthogammarus grewingkii endemic to lake Baikal. PLoS ONE 7: 1–9.
Milchunas, D. G., O. E. Sala & W. K. Lauenroth, 1988. A generalized model of the effects of grazing by large herbivores on grassland community structure. American Naturalist 132: 87–106.
Mordukhay-Boltovskoy, F., 1964. Caspian Fauna in Fresh Waters outside the Ponto-Caspian Basin. Hydrobiologia 23: 159–164.
Neilson, M. E. & C. A. Stepien, 2009. Escape from the Ponto-Caspian: evolution and biogeography of an endemic goby species flock (Benthophilinae: Gobiidae: Teleostei). Molecular Phylogenetics and Evolution. 52: 84–102. https://doi.org/10.1016/j.ympev.2008.12.023.
Pellan, L., V. Médoc, D. Renault, T. Spataro, & C. Piscart, 2015. Feeding choice and predation pressure of two invasive gammarids, Gammarus tigrinus and Dikerogammarus villosus, under increasing temperature. Hydrobiologia.
Phillips, J. G., & T. J. Hagey, 2022. Rapid morphological shifts in a co-invaded assemblage of lizards. Research Square Preprint: 1–19.
Pigot, A. L., C. Sheard, E. T. Miller, T. P. Bregman, B. G. Freeman, U. Roll, N. Seddon, C. H. Trisos, B. C. Weeks & J. A. Tobias, 2020. Macroevolutionary convergence connects morphological form to ecological function in birds. Nature Ecology and Evolution 4: 230–239. https://doi.org/10.1038/s41559-019-1070-4.
Platvoet, D., J. T. A. Dick, N. Konijnendijk & G. Van Der Velde, 2006. Feeding on micro-algae in the invasive Ponto-Caspian amphipod Dikerogammarus villosus (Sowinsky, 1894). Aquatic Ecology 40: 237–245.
Platvoet, D., G. Van Der Velde, J. T. A. Dick & S. Li, 2009. Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda)—Amphipod Pilot Species Project (AMPIS) Report 5. Crustaceana 82: 703–720.
Podwysocki, K., A. Desiderato, T. Mamos, T. Rewicz, M. Grabowski, A. Konopacka & K. Bącela-Spychalska, 2024. Recent invasion of Ponto-Caspian amphipods in the Masurian Lakeland associated with human leisure activities. NeoBiota 90: 161–192.
Premate, E., Š Borko, T. Delić, F. Malard, L. Simon & C. Fišer, 2021. Cave amphipods reveal co-variation between morphology and trophic niche in a low-productivity environment. Freshwater Biology 66: 1876–1888.
R Core Team, 2023. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna.
Rewicz, T., M. Grabowski, C. Macneil & K. Bącela-Spychalska, 2014. The profile of a ’ perfect ’ invader—the case of killer shrimp, Dikerogammarus villosus. Aquatic Invasions 9: 267–288.
Rewicz, T., R. A. Wattier, T. Rigaud, K. Bącela-Spychalska & M. Grabowski, 2015a. Isolation and characterization of 8 microsatellite loci for the “killer shrimp’’, an invasive Ponto-Caspian amphipod Dikerogammarus villosus (Crustacea: Amphipoda). Molecular Biology Reports 42: 13–17.
Rewicz, T., R. Wattier, M. Grabowski, T. Rigaud & K. Bącela-Spychalska, 2015b. Out of the Black Sea: phylogeography of the invasive killer shrimp Dikerogammarus villosus across Europe. PLoS ONE 10: e0118121. https://doi.org/10.1371/journal.pone.0118121.
Rewicz, T., A. Konopacka, K. Bącela-Spychalska, M. Özbek & M. Grabowski, 2016. First records of two formerly overlooked Ponto-Caspian amphipods from Turkey: Echinogammarus trichiatus (Martynov, 1932) and Dikerogammarus villosus (Sovinsky, 1894). Turkish Journal of Zoology 40: 328–335.
Rewicz, T., R. Wattier, T. Rigaud, M. Grabowski, T. Mamos & K. Bącela-Spychalska, 2017. The killer shrimp, Dikerogammarus villosus, invading European Alpine Lakes: a single main source but independent founder events with an overall loss of genetic diversity. Freshwater Biology 62: 1036–1051.
Richter, L., L. Schwenkmezger, J. Becker, C. Winkelmann, C. Hellmann & S. Worischka, 2018. The very hungry amphipod: the invasive Dikerogammarus villosus shows high consumption rates for two food sources and independent of predator cues. Biological Invasions 20: 1321–1335.
Šidagytė‐Copilaş, E., & D. Copilaş‐Ciocianu, 2024. Climatic niche differentiation between native and non‐native ranges is widespread in Ponto‐Caspian amphipods. Freshwater Biology 277–287.
Šidagyte, E., V. Razlutskij, A. Alekhnovich, A. Rybakovas, M. Moroz, V. Šniaukštaitė, G. Vaitonis & K. Arbačiauskas, 2017a. Predatory diet and potential effects of Orconectes limosus on river macroinvertebrate assemblages of the southeastern Baltic Sea basin: Implications for ecological assessment. Aquatic Invasions 12: 523–540.
Šidagytė, E., S. Solovjova, V. Šniaukštaitė, A. Šiaulys, S. Olenin & K. Arbačiauskas, 2017b. The killer shrimp Dikerogammarus villosus (Crustacea, Amphipoda) invades Lithuanian waters, South-Eastern Baltic Sea. Oceanologia 59: 85–91.
Sotka, E. E., A. W. Baumgardner, P. M. Bippus, C. Destombe, E. A. Duermit, H. Endo, B. A. Flanagan, M. Kamiya, L. E. Lees, C. J. Murren, M. Nakaoka, S. J. Shainker, A. E. Strand, R. Terada, M. Valero, F. Weinberger & S. A. Krueger-Hadfield, 2018. Combining niche shift and population genetic analyses predicts rapid phenotypic evolution during invasion. Evolutionary Applications 11: 781–793.
Suarez, A. V. & N. D. Tsutsui, 2008. The evolutionary consequences of biological invasions. Molecular Ecology 17: 351–360.
Väinölä, R., J. D. S. Witt, M. Grabowski, J. H. Bradbury, K. Jażdżewski & B. Sket, 2008. Freshwater Animal Diversity Assessment. Hydobiologia 595: 241–255.
Valen, L. V., 1965. Morphological variation and width of ecological niche. The American Naturalist 99: 377–390.
Van der Velde, G., S. Rajagopal, B. Kelleher, I. B. Muskó & A. B. De Vaate, 2000. Ecological impact of crustacean invaders: general considerations and examples from the Rhine River. Biodiversity Crisis and Crustacea 12: 3–34.
van Riel, M. C., G. Van Der Velde, S. Rajagopal, S. Marguillier, F. Dehairs & A. Bij de Vaate, 2006. Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565: 39–58.
Watling, L., 1993. Functional morphology of the amphipod mandible. Journal of Natural History 27: 837–849. https://doi.org/10.1080/00222939300770511.
Wattier, R. A., E. R. Haine, J. Beguet, G. Martin, L. Bollache, I. B. Muskó, D. Platvoet & T. Rigaud, 2007. No genetic bottleneck or associated microparasite loss in invasive populations of a freshwater amphipod. Oikos 116(11): 1941–1953.
Wesselingh, F. P., T. A. Neubauer, V. V. Anistratenko, M. V. Vinarski, T. Yanina, J. J. ter Poorten, P. Kijashko, C. Albrecht, O. Y. Anistratenko, A. D’Hont, P. Frolov, A. M. Gándara, A. Gittenberger, A. Gogaladze, M. Karpinsky, M. Lattuada, L. Popa, A. F. Sands, S. van de Velde, J. Vandendorpe & T. Wilke, 2019. Mollusc species from the Pontocaspian region—an expert opinion list. ZooKeys 2019: 31–124.
Willi, Y., J. Van Buskirk & A. A. Hoffmann, 2006. Limits to the adaptive potential of small populations. Annual Review of Ecology, Evolution, and Systematics 37: 433–458.
Worischka, S., L. Richter, A. Hänig, C. Hellmann, J. Becker, P. Kratina & C. Winkelmann, 2018. Food consumption of the invasive amphipod Dikerogammarus villosus in field mesocosms and its effects on leaf decomposition and periphyton. Aquatic Invasions 13: 261–275.
Záhorská, E., M. Šúrová & M. Balážová, 2023. Morphological variability in a successful invasive species originating from habitats experiencing different levels of disturbance. Journal of Vertebrate Biology 72: 23031.
Zhao, Y., C. Galvão & W. Cai, 2021. Rhodnius micki, a new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys 1012: 71–93.
Acknowledgements
We would like to thank Eglė Šidagytė-Copilaş (Nature Research Centre in Vilnius) for help and advice in the statistical analyses. We are grateful to Carl Smith, Richard Bailey, Stephen Venn, Krzysztof Pabis and Tomasz Mamos (University of Lodz) for constructive discussion that enhanced the quality of this manuscript. We would like to thank Jarosław Kobak and Łukasz Jermacz (Nicolaus Copernicus University in Torun) for help in the project proposal preparation and implementation of interesting conceptions. We are grateful to Michał Grabowski (University of Lodz) for advice during project proposal preparation and suggestions during the project implementation. Thanks are also due to two anonymous reviewers for their invaluable contributions to the improvement of this manuscript.
Funding
The fellowship for the KP allowing for the study performance was founded by the University of Lodz within internal funds (IDUB Grant). The samples were collected within Projects 2018/31/D/NZ8/03061 and 2011/03/D/NZ8/03012 funded by Polish National Science Centre, N N304 350139 and N N304 081535 funded by Polish Ministry of Science and Education; Ponto-Caspian amphipods in the Baltic region: niche changes and functional comparison with local species (AMPHIBALT) funded by Research Council of Lithuania as well as field expeditions covered by internal funds of the University of Lodz.
Author information
Authors and Affiliations
Contributions
KP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Funding acquisition, Project administration, Visualization, Writing—original draft. KBS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing—review & editing. AD: Conceptualization, Methodology, Writing—review & editing, TR: Conceptualization, Resources, Supervision, Writing—review & editing. DCC: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Katya E. Kovalenko
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Sidinei M. Thomaz, Cécile Fauvelot, Lee B. Kats, Jonne Kotta & Fernando M. Pelicice / Aquatic Invasive Species IV
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Podwysocki, K., Bącela-Spychalska, K., Desiderato, A. et al. Environment, intraspecific lineages and geographic range jointly shape the high morphological variability of Dikerogammarus villosus (Sowinsky, 1894) (Crustacea, Amphipoda): a successful aquatic invader across Europe. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05565-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05565-8