Abstract
Several rodent genera radiated in Ethiopian highlands during Pleistocene, giving rise to species specialized to different elevation zones. The zonation is accompanied by vegetation change from savannahs or forests in lower elevations, through the mid-elevation belt of ericaceous shrublands to Afroalpine moorlands on the mountain tops. Such evolution on elevation gradient is expected to promote phenotypic adaptations and several genera are known to include highly distinctive high-elevation specialists. In this study, we systematically examined shape and size differentiation of skull and mandible in three genera, Lophuromys, Arvicanthis and Stenocephalemys, each including from six to eight species inhabiting different elevation zones on both sides of the Great Rift Valley. Employing computed tomography, three-dimensional virtual models, landmark-based geometric morphometrics and a powerful toolkit of partial least squares analysis, we demonstrated the elevation-related differentiation is a prominent feature of skull and mandible shape variation in all three genera. We identified the parts of skull and mandible which change most between the successive zones. These include, notably, orbits and insertion areas of masticatory muscles, which suggests adaptive shifts in visual perception and diet. We also showed that while the same parts are modified in all three genera, they are modified in different ways and to a different degree. The evidence for size differentiation was mixed despite the universal trend of larger size in higher elevations. Our study confirms the high-elevation specialists represent the most distinctive forms, which is especially true for the species from Bale and Arsi Mountains, East of the Great Rift Valley.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On mountain slopes, climate and vegetation change markedly over short distances, creating strong selective pressures potentially resulting in directional phenotypic change and ecological speciation (Körner, 2007; Keller et al., 2013; Bertrand et al., 2016a, b). This evolutionary outcome is favoured also by stability of the gradients because elevation zones tend to shift rather than disappear when subjected to large-scale climatic changes (Fjeldsa et al., 2007; Mosbrugger et al., 2018; Graham et al., 2018; Couvreur et al., 2021). At the same time, a rugged landscape with peaks separated by deep valleys limits dispersal of high-elevation specialists. The eventual outcome of this separation may be parallel evolution of the same phenotype on different mountain ranges (Suzuki et al., 2018), but also accumulation of reproductive incompatibilities between populations isolated by non-favorable lowland habitats, whose admixture is limited to episodes of zonal shifts so large they cause downhill reconnection of high-elevation habitats (Feijó et al., 2019; Ohsawa & Ide, 2008).
The Ethiopian highlands represent a prime example of a landscape supporting this kind of diversification (Gotelli et al., 2004; Belay & Mori, 2006; Ehrich et al., 2007; Kebede et al., 2007; Evans et al., 2011; Freilich et al., 2014; Bryja et al., 2019a). Their slopes can overcome the elevation gain of 2000 m over distances of 10–20 km, which means substantial environmental and vegetation change takes place in rapid succession (van Breugel et al., 2015; Friis et al., 2010; Asefa et al., 2020). The mountain ranges are surrounded by lowland savannahs (900–1900 m.a.s.l.) with dominating Acacia-Commiphora deciduous bushlands in the Great Rift Valley, the Afar Triangle and south-eastern Ethiopia, and the Combretum wooded grasslands in western Ethiopia. The foothills and the lower part of the mountain slopes (500–2600 m.a.s.l.) are dominated by various types of forests with evergreen bushes and trees e.g., from genera Ilex, Ocottea, Nuixa and Olea. They are found especially in south-western Ethiopia but occur in small patches also in lower slopes of Bale and Arsi Mts. and in several other remnant fragments in northern Ethiopia. The forest zone is replaced by the evergreen montane and grassland vegetation in higher elevation (1800–3500 m.a.s.l.), with a transitional ericaceous belt of heathlands and shrublands dominated by tree heaths (Erica). Very often it forms a mosaic with the swampy grasses and mats of mosses, and in many places, this is the elevation which is most affected by human activities. The highest parts of mountains (3200–4500 m.a.s.l.) are covered by an open vegetation of Afroalpine grasslands and moorlands, which is mostly herbaceous with sparse shrubs and woody plants (e.g., from genera Lobelia or Helichrysum). They are best developed in the highest elevation of the Simien and Bale Mts.
In this zonal landscape multiple rodent genera radiated into species occupying different habitats and sometimes showing conspicuous phenotypic changes (Bryja et al., 2019a). The narrow-headed rats (Stenocephalemys) occupy all three montane vegetation zones with different species being specialized to forests, transitional shrublands, and Afroalpine moorland (Bryja et al., 2018; Mizerovská et al., 2020). Another genus which adapted to the whole variety of mountain habitats is the brush furred rat (Lophuromys). Notably, some of its species specialized even to the treeless Afroalpine meadows, although the genus usually requires at least minimum tree cover (Lavrenchenko et al., 2007). Finally, the grass rats (Arvicanthis) inhabit exclusively open habitats, but they are found in lowland savannahs as well as in the Afroalpine zone (Bryja et al., 2019b).
The present-day communities were shaped also by topographical fragmentation of Ethiopian highlands. Most importantly, the highlands are divided in two major blocks by the Great Rift Valley (GRV) running in approximately north–south direction. Its bottom was even in more humid periods of Pleistocene covered with savannah and lakes of varying extent (Lamb et al., 2004; Marshall et al., 2009). As such, GRV represents a substantial long-term migration barrier for most species inhabiting the mountain slopes. This is apparent from the composition of communities found east and west to GRV and presence of the east–west subdivision in the phylogeographic structure of multiple mountain taxa (Otomys—Taylor et al., 2011; Lophuromys—Lavrenchenko et al., 2007; Komarova et al., 2021; Arvicanthis—Bryja et al., 2019b; Stenocephalemys Bryja et al., 2018; Mizerovská et al., 2020; Tachyoryctes—Šumbera et al., 2018).
There is some evidence of morphological evolution taking place on the elevation gradient. In Stenocephalemys, ordination of species according to the skull shape matches the zonation (Fadda & Corti, 2000; Mizerovská et al., 2020). Species of the forests occupy one extreme, Afroalpine species the other and species of transitional habitats are found in between. Skull of S. albocaudatus is outstanding in its shape with powerful zygomatic arches and very narrow inter-orbital constriction allowing shift of the eyes to the top of the head. S. albocaudatus is also the most distinctive species in external body measurements (Mizerovská et al., 2020). In Lophuromys, L. melanonyx from the top of Bale Mts. was described as morphologically outlying to all other studied Ethiopian populations of the genus (Bekele & Corti, 1994). In Arvicanthis, skull shape variation was studied by Fadda and Corti (2001), who found A. blicki, an Afroalpine specialist, characterized by “extreme and unique shape changes”, including a downward twist of the rostrum and an enlargement of the orbit.
Based on the association of morphologically distinctive species with the highest elevation zone, we hypothesize that in all these genera, skull morphology successively changes along the elevational gradients. The genera Lophuromys, Arvicanthis and Stenocephalemys are especially suitable for testing of this hypothesis. First, they inhabit the whole range of elevation zones (low, middle, and high). Second, they can be considered independent evolutionary replicates. Their ancestors are as old as ~ 11 Ma (Arvicanthis/Stenocephalemys) and ~ 19 Ma (Arvicanthis/Lophuromys) and phylogenetically they are nested within lineages inhabiting the widest variety of habitats (Aghová et al., 2018; Mikula et al., 2021; Nicolas et al., 2021). Moreover, habitat specializations also evolved repeatedly within the genera Stenocephalemys and Lophuromys, because their high-elevation species from the opposite sides of GRV are not in sister relationship (Komarova et al., 2021; Mizerovská et al., 2020).
We focused on the shape of skull and mandible of Lophuromys, Arvicanthis and Stenocephalemys. In each genus, we looked for shape features changing progressively in the direction of increasing elevation, and we assessed whether they change by the same extent between any two adjacent elevation zones. Finally, we compared shape changes observed in different genera and figured out in what aspects they are congruent. An idealized picture we expected to see was that of mid-elevation species being intermediate to low- and high-elevation species in all three genera and the associated shape changes being largely identical across them.
Material and Methods
Taxon Sampling
In total we analyzed 101 individuals representing three genera Lophuromys (n = 21), Arvicanthis (n = 28) and Stenocephalemys (n = 52) and almost all their species living in Ethiopia. The specimens are deposited in the collections of the Institute of Vertebrate Biology of the Czech Academy of Sciences (84 individuals) and the Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences (17 individuals). For every specimen we scanned both skull and mandible, but due to damage of some specimens, the final sample sizes differed slightly (100 skulls and 97 mandibles). Lophuromys was represented by eight out of nine species living in Ethiopia (Komarova et al., 2021), namely by L. brevicaudus, L. brunneus L. cherchernsis, L. chrysopus, L. flavopunctatus, L. melanonys, L. pseudosikapusi and L. simensis, from Arvicanthis seven out of its eight Ethiopian species were included, namely A. abyssinicus, A. blicki, A. niloticus, A. niloticus ‘C2–C4’, A. raffertyi, A. saturatus and A. somalicus (all names are sensu Bryja et al., 2019b, including the informal A. niloticus ‘C2–C4’, a monophyletic cluster non-sister to the rest of A. niloticus in some phylogenies). In Stenocephalemys we included all six species S. albipes, S. albocaudatus, S. griseicauda, S. ruppi, S. sokolovi and S. zimai. For sample size of each species, see Table 1.
The species were assigned to the three elevation zones: low (either forest or savannah), middle (ericaceous belt) and high (Afroalpine moorland). The low-elevation group comprises species inhabiting quite different biomes: mountain forest in Lophuromys and Stenocephalemys and bushland savannah in Arvicanthis. The common theme here, however, is the difference in tree cover, which is still relatively high in the bushland savannah compared to entirely treeless Afroalpine moorlands. More specifically, (1) the typical rodent community is found in forests of south-western Ethiopia and consists of S. albipes, S. ruppi, L. chrysopus, L. brunneus and L. pseudosikapusi. The savannah in the low elevation of the Rift Valley is inhabited by relatively high diversity of Arvicanthis species (A. raffertyi, A. somalicus, A. niloticus, A. niloticus ‘C2-C4’); (2) the mid-elevation habitats have characteristic rodent fauna at both north-western (S. sokolovi, A. saturatus, L. flavopunctatus) and south-eastern (S. griseicauda, L. brevicaudus, L. chercherensis) blocks of Ethiopian highlands; (3) also Afroalpine communities have characteristic fauna in these two blocks, represented, respectively, by the Simien (S. zimai, A. abyssinicus, L. simensis) and Bale Mts. (S. albocaudatus, A. blicki, L. melanonyx).
The classification was based on the preferred habitats and elevational distribution of the species (see the references above, Bryja et al., 2019a; Craig et al., 2020). For its summary and illustration of the habitats, see Table 1 and Fig. 1. For complete account of the material, including taxonomic classification, habitat, elevation, locality, longitude, and latitude of each specimen, see Supplementary Material 1. Geographical distribution of sampling sites is shown in Fig. S1 (Supplementary Material 2).
Micro-Computed Tomography
The analysis was conducted on virtual three-dimensional models of skulls and mandibles produced by means of micro-computed tomography. Skulls and mandibles were scanned separately in SkyScan 1176 High-Resolution in-vivo X-ray Microtomograph (Bruker, Belgium) under resolution of 18 µm with 0.5 mm aluminum filter (voltage = 50 kV, current = 500 µA, exposure time = 250 ms with 3 times averaging and full circle scan). NRecon 1.7.1 (Bruker, Belgium) was used to create digital tomographic sections (intensities 0.0035–0.1500 AU, smoothing = 10, ring artifact correction = 2, beam hardening = 36%). Automatic separation of the scanned object was performed by Otsu method (Cheriet et al., 1998). PLY 3D models were created in CT analyser 1.17.7.2 (Bruker, Belgium). The scanning was performed in the Czech Centre for Phenogenomics of the Biotechnology and Biomedicine Centre BIOCEV.
Geometric Morphometrics
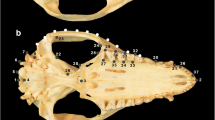
Skull and mandible size and shape were analysed by methods of landmark-based geometric morphometrics (Bookstein, 2018; Dryden & Mardia, 2016). It was thus described by a set of points placed on mutually corresponding (anatomically homologous) positions. The point positions are recorded in three-dimensional coordinate system and their whole configuration serves as a descriptor of size and shape. Three types of landmarks were used: fixed landmarks (115 on the skull, 26 on the mandibles) and curve semilandmarks (710 on the skull, 113 on the mandibles) (Fig. 2). While fixed landmarks are placed on anatomically well-defined positions and thus homologous on its own (Bookstein, 1991), in semilandmarks the notion of homology applies primarily to whole curves (Bookstein, 1997; Gunz et al., 2005). Semilandmarks are, therefore, recorded in a fixed (i.e., mutually corresponding) order, but their precise positions are to be algorithmically optimized to reflect curve homology. Anatomic definitions of the fixed landmarks are in Table S1 (Supplementary Material 2).
Landmarks describing size and shape of skull and mandible. Fixed landmarks (115 on the skull, 26 on the mandibles) are shown in red and semilandmarks (710 on the skull, 113 on the mandibles) in green. Their positions are displayed in three complementary views (dorsal, lateral and ventral) and the numbers refer to the landmark list in Table S1
As the position and orientation of skulls and mandibles in the coordinate system is arbitrary and size and shape differences are confounded, the coordinates must be standardized prior to any statistical analysis. This is achieved by Generalized Procrustes analysis (GPA), an iterative superimposition procedure aligning a set of shapes as closely as possible (Gower, 1975; Rohlf & Slice, 1990). In GPA, configurations are translated to have their centroids at the origin, scaled to unit size and rotated to optimize their mutual fit under least-squares criterion. In addition, semilandmarks are slid to minimize bending energy associated with deformation of the curves to their counterparts on the average configuration (Bookstein, 1997; Gunz et al., 2005). It means to reduce non-affine (locally disproportional) shape differences among the curves under the constraint imposed to sliding by local tangent directions of the curves. In effect, GPA algebraically separates size of the configurations, measured as centroid size (Bookstein, 1991), and their shape, captured by Procrustes aligned coordinates. Mathematically, Procrustes aligned coordinates are found in a curved (non-Euclidean) space and thus they are post hoc projected into a tangent linear space (Rohlf, 1999).
The studied structures are both symmetric, but in different ways: while the skull is symmetric internally, the mandible consists of two counterparts (they show object and matching symmetry, respectively, in terminology of Klingenberg et al., 2002). We assumed asymmetries to result from measurement error or stochastic variation unrelated to our research questions (for justification, see Fig. S2). We therefore focused on symmetric variation only. For the skull, we did it by retaining the symmetric component of variation in Procrustes shape coordinates, while for the mandible we reflected the left part and if the right part was present too, we aligned and averaged the counterparts prior to GPA. In 13 skulls and 15 mandibles several landmarks were missing, which is not tolerated by GPA. We therefore imputed these missing data prior to any further analysis. We superimposed separately any concerned mandibles with its reflected counterpart or any concerned skull with its mirror image, supplied the missing landmarks from the counterpart and transformed the coordinates back. This was not possible for landmarks on the skull midplane or those missing on both sides and such skulls or mandibles were excluded from the study.
Landmark digitization was performed Stratovan Checkpoint software (Stratovan Corporation, https://www.stratovan.com/products/checkpoint). GPA of skulls and mandibles was performed in packages Morpho (Schlager, 2017) and geomorph (Adams et al., 2020) for R (R Core Team, 2021). Missing data were imputed, and the results visualized using R package rgl (Adler & Murdoch, 2020) and custom R functions available at https://github.com/onmikula/gmtools.
Statistical Analysis
Shape variation in each of the data sets (skull and mandible) was first subjected to explorative analysis and dimension reduction by principal component analysis (PCA). For all subsequent analyses, a limited number of principal components was retained, namely 20, which was the minimum number of individuals per genus. In both skulls and mandibles, the retained PCs account for 92% of total shape variability. The purpose was to avoid statistical pathologies caused by the number of observations being much lesser than dimensionality of data (\(n \ll p\) condition, Hastie et al., 2001; Cardini et al., 2019). These components were projected back to the Procrustes shape coordinates so the data matrices had large number of columns (2475 in skulls, 390 in mandibles), but their actual dimensionality (= rank of their covariance matrices) was 20.
In this reduced space we looked for shape features that covary with elevation gradation. It means the features that change from low-elevation to mid-elevation species and then again from mid-elevation to high-elevation species. These features were identified by means of partial least squares (PLS) analysis, which extracts components of shape variation that are predictive of the response variables, in this case encoding the elevation gradation. Each of these components is a linear combination of shape variables (GPA-standardized x, y, z landmark coordinates) and thus indicate shape difference: a coordinated shift of landmarks and semilandmarks in three dimensions of the physical space. The response variables are embedded in a matrix (denoted A) whose rows represent low-, mid- and high-elevation, respectively, and whose columns represent different contrasts between the zones:
In PLS analysis, the components come in pairs: every shape component has its elevation counterpart, which is, in turn, a linear combination of the elevation variables (columns of the matrix A). Coefficients defining the shape component capture shape features predictive of a certain gradation pattern, whose nature is encoded in the coefficients of the corresponding elevation component. Namely, the coefficient associated with the first elevation variable (the first column of A) expresses magnitude of proportional change between successive elevation zones, because its value for the mid-elevation (0.5) is an average of low- and high-elevation values (0.0 and 1.0, respectively). The coefficients associated with the second and third columns of A express disproportionality of the change. They may decrease the predicted magnitude of change between low and mid elevation relative to change between mid and high elevation (or possibly even to swap the expected ordering of the zones). For instance, if the coefficient of the second variable is high and positive, it means the correlated shape change involves a difference between low- and mid-elevation species (both coded by 1.0) relative to high-elevation ones (coded by 0.00).
We fitted PLS by NIPALS algorithm (Wold, 1975), an iterative procedure returning each time a pair of components that co-vary as much as possible. Every next pair is fitted to variation, which is left after projecting out variation accounted for by the preceding pairs. We used “PLS2” variant of the algorithm, which distinguishes predictor and response variables (Rosipal & Krämer, 2005). The algorithm was implemented in R function ‘nipals’ also available at https://github.com/onmikula/gmtools.
PLS was conducted separately in each genus on the species mean shapes, which makes the analysis robust to unbalanced sample size and possible differences in intra-specific variation patterns. The individual shapes were then projected on the resulting PLS components (like in between-group PCA; Cardini et al., 2019) to visualize overlap of the species along the lines of the predicted shape change. The species mean shapes were centred prior to PLS analysis and their elevation variables scaled to unit variance to account also for unequal number of species from different elevation zones. In each genus, we visualized the elevation-associated shape differentiation by a scatter plot of the first two PLS shape components with both species means and individual scores displayed. It shows how much this differentiation separates elevation groups, but also their constituent species. Then, we examined shape difference associated with the first PLS shape component and gradation of elevation zones predicted by its elevation counterpart. The direct comparison of genus-specific trends was achieved by taking each genus-specific PLS1 shape component in turn, deforming the overall mean shape in a way prescribed by the component, and comparing the resulting shapes.
As PLS comes with no test of significance, we also used the analysis of variance using distance matrices as implemented in adonis2 function of R package vegan (Oksanen et al., 2022). The fitted linear model was fully factorial with genus, elevation zone and their interaction as predictors and Euclidean distance matrix between PC1-20 scores of species mean shapes as a response. The significance was tested with a permutation test (99,999 iterations). The elevation zones were encoded as in the first column of the matrix A and hence the proportionality of shape change was assumed.
In addition, we also compared skull and mandible size among elevation zones in each genus. The elevation zones were treated as levels of an ordered factor and significance of the difference between them was tested by a linear mixed effects model with elevation zone as the fixed effect and species as a random effect. The models were fitted using the maximum likelihood criterion in R package nlme (Pinheiro et al., 2020). The model including the elevation zone was compared to the null (intercept-only) model by Akaike Information Criterion (AIC; Akaike, 1974). In models with \(\Delta {\text{AIC}} > 2\) we performed Tukey’s honest significance post hoc tests (Tukey, 1949), as implemented in R package multcomp (Hothorn et al., 2008). Finally, we fitted a model with the fixed effects of elevation and genus and tested for significance of their interaction to detect possibly discordant trends in size. Size variation was visualized in R using packages lattice (Sarkar, 2008) and latticeExtra (Sarkar & Andrews, 2019).
Results
The three genera are clearly distinct in their skull and mandible shape (Fig. 3) on the first principal plane defining the shape space. In the skull, the first principal component (PC1) separates Arvicanthis and Stenocephalemys (Murinae) from Lophuromys (Deomyinae), whereas PC2 singles out Arvicanthis. In the mandible, all the genera are separated along PC1 and Stenocephalemys stands apart along PC2. The figure also suggests elevation gradation to account for a part of shape variation, because in both datasets the ordination of species in at least one genus clearly follows the zonation pattern. For clarity, Fig. 3 shows species means, although PCA was conducted on individual shapes.
The first two principal components of skull and mandible shape variation in all three genera. For clarity, each species is represented by its centroid. Points shape indicates the genus (square = Lophuromys, circle = Arvicanthis, rhomb = Stenocephalemys), colour indicates elevation zone (blue = low-elevation, yellow = mid-elevation, red = high-elevation)
The analysis of variance showed both skull and mandible shape being significantly different between genera (P < 0.0001) and the difference accounted for a large proportion of total variation (R2 = 0.74 and 0.75, respectively). In skull, the effect of elevation zone was also highly significant (P = 0.0001), but the response had also genus-specific component as evident from the significance of the genus × elevation interaction (P = 0.0138). The general effect of the elevation zone was larger, however, than the genus-specific effect (R2 = 0.10 and 0.05, respectively). In mandible, the main effect was marginally non-significant (P = 0.0532), whereas the interaction reached significance (P = 0.0137). Here, the main term accounted for less variation than the interaction term (R2 = 0.03 and 0.07, respectively).
PLS analysis of skull shape variation highlights the importance of elevation gradation. Individuals of high-elevation species are always separated from those of low-elevation species and mid-elevation ones are in between on the two first PLS components (Fig. 4). The first PLS component (PLS1) explained a substantial proportion of variation. At the level of species means, it was 0.44 in Lophuromys, 0.72 in Arvicanthis and 0.80 in Stenocephalemys, and at the individual level the numbers were 0.31, 0.46 and 0.57, respectively. These proportions were much lower with PLS2 (see Table 2), but the second component was still crucial for separating species belonging to different elevation zones, which indicates presence of an additional shape change associated with the gradation. In the analysis of mandible shape the first component also dominated, although less remarkably. The proportions of variation explained in Lophuromys, Arvicanthis and Stenocephalemys were 0.46, 0.48 and 0.54 at the species level and 0.33, 0.26 and 0.22 at the individual level, respectively (Table 2).
The first two PLS components corresponding to elevational trends in shape variation as estimated separately for skull and mandible and in each of the genera. Colour code is the same as in Fig. 3 (blue = low-elevation, yellow = mid-elevation, red = high-elevation). The filled symbols represent species means, open symbols individual shapes. The envelopes enclose symbols belonging to the species whose name is indicated by the closest text of the corresponding colour. Arrows point towards mean or individual shapes that are either outlying (found in other species’ envelope). In few cases, envelope was not drawn for the species, and the assignment is indicated just by arrows
The ordination plots (Fig. 4) show there was a single high-elevation species per genus with an extremely differentiated skull morphology, namely L. melanonyx, A. blicki and S. albocaudatus. Interestingly, all these species were from the eastern bloc of the highlands. Skull differentiation of their western counterparts (L. simensis, A. abyssinicus and S. zimai) was less pronounced, but the mandible shape differentiation was comparable in both high-elevation species of Arvicanthis and Stenocephalemys. Among the low-elevation species, S. albipes can be identified as the extreme one due to its position in both skull and mandible shape ordination plot. The syntopic S. ruppi was separated from it by its skull shape and shifted more towards the mid-elevation species. In Arvicanthis, just A. somalicus was largely separated from the other lowland species but not in a way that could be clearly linked to the zonal differentiation. In Lophuromys, there is no easily interpretable ordination of low-elevation species. The mid-elevation species of Stenocephalemys were clearly separated in the direction of zonal gradation, S. griseicauda being closer to the high-elevation species in in both skull and mandible shape. Similarly, in Lophuromys, the mid-elevation species were quite distinctive, but their ordination was different between the skull and the mandible. Inspection of the predicted elevation scores shows that in Arvicanthis and Stenocephalemys, PLS targeted approximately even zonal gradation, because the differences between elevation scores predicted for low- and mid- elevation species are comparable to those between mid- and high-elevation species. Only in Lophuromys, the nature of detected trend was more in contrast between high-elevation species and the rest (Table 3). It was pointed out that the ample number of semilandmarks can bias analyses like this one (Cardini et al., 2019), but it was not the case here as the ordination plots were very similar to Fig. 4 when based on fixed landmarks only (Fig. S3).
Skull and mandible shape differences associated with zonal gradation in particular genera are shown by wireframe plots, which contrast differences predicted for the minimum and maximum species means of PLS1 scores (Figs. 5, 6). These predicted shapes reflect which way the shape changes with increasing elevation and the amount of change. Technically, they reflect both direction and magnitude of shift in the multivariate shape space. Genus-specific trends are compared by PLS1-based unit deformations of the common consensus, which reflects just the direction of the shift (Figs. 7, 8, S4 and S5).
Skull shape differences accounted for by PLS1, i.e., the main elevational trend. Wireframe plots contrast shapes corresponding to the minimum and maximum species mean PLS1 scores. In all plots, the low-elevation (blue) corresponds to the minimum and high-elevation to the maximum red. The same predicted shapes are shown in three views (dorsal, lateral, and ventral)
Mandible shape differences accounted for by PLS1, i.e., the main elevational trend. Wireframe plots are arranged as in Fig. 5, but only a single (lingual) view is given
The main skull shape elevational trend in the three genera shown on the consensus (GPA grand mean) shape in the dorsal, lateral, and ventral view. The intensity of red colour indicates the local average distances of the three PLS1-predicted shapes from the consensus (A) and the local average distances between these three genera (B)
The main mandible shape elevational trend in the three genera shown on the consensus (GPA grand mean) shape in the lingual view. The intensity of red colour indicates the local average distances of the three PLS1-predicted shapes from the consensus (A) and the local average distances between these three shapes (B)
In Lophuromys, high-elevation species have narrower inter-orbital constriction than the low-elevation ones and narrower braincase, especially at its top (frontal and parietal bones). Their skull is flatter in the frontal and parietal area and shorter in the occipital area. The maxillary region is elongated in the rostral direction, which involves elongation of the maxillary fossa adjacent to the infraorbital foramen, anterior shift of palatine foramens, more anterior origin of the zygomatic plate and more anterior position of incisors. Nasal bones, on the contrary, are longer at their posterior end. Their zygomatic arch is considerably wider and molars lower relative to the upper edge of the orbit. The angular process is shorter and the coronoid process stubbier in high elevation species.
In Arvicanthis, the inter-orbital constriction is narrower in high-elevation species and their braincase is narrower and flatter in the parietal area and shorter in the occipital one. The maxillary fossa is elongated in the rostral direction and palatine foramens as well as incisors are shifted in the same way. Zygomatic plate is higher than in low-elevation species and zygomatic arch is shifted upwards and tilted towards the midplane. Nasal bones of high-elevation species are shorter at their posterior end. The lower edge of the high-elevation mandible has a more cut-out shape and its angular process is slender and longer.
In high-elevation species of Stenocephalemys, the skull is more arched, its rostrum is elongated, and its occipital part shortened. The inter-orbital constriction gets very narrow, and the top of the braincase gets much narrower compared to its base, so the braincase is rather triangular at its coronal cross-section. The anterior part of zygomatic arches is much wider in the sense of left–right distance, but also the bones forming this structure, namely zygomatic process of maxilla and the zygomatic bone, are much wider. The zygomatic plate is larger than in low-elevation species. The rostral elongation of the skull shifts the incisors forward, although the angle their form with the coronal plane is unchanged. Also, the maxillary fossa is enlarged and shifted forward and the same applies to incisive foramens. The nasal bones, on the contrary and just slightly longer at their posterior end. The mandible of high-elevation species is narrower in its posterior part and hence the distance between tips of the coronoid and angular process is shorter.
The comparison of unit deformations along PLS1 (“high-elevation shapes”) reveals the same parts of the skull and mandible were involved in the elevation-related differentiation in all three genera, but the actual shape changes of these parts were often different. In particular, there is an important specific component of variation that is typical of each genus for these parts. For the skull, this observation is summarized in Fig. 7. Its left panel (Fig. 7A) highlights the parts that are modified in high-elevation shapes of all genera, while the right panel (Fig. 7B) highlights those parts that vary among these high-elevation shapes. It follows the structure highlighted on the left but not on the right is changed in the same way in all three genera. The direct comparison of the high-elevation shapes superimposed on the consensus (= GPA grand mean shape) is provided is Fig. S4 (see Supplementary Material 2).
An universally shared feature is the change of the rostral region, which is always elongated so the incisors are shifted forward, and the maxillary fossa as well as the incisive foramens are enlarged for species inhabiting high elevation. The tip of the nasal is not shifted forwards, however, so the high-elevation species can be described as having a bit stronger overbite. At their posterior edge the nasal bones are shortened in Arvicanthis but enlarged in Stenocephalemys and Lophuromys. The interorbital constriction and parietal region of the braincase are narrowing in all genera, but in different ways. Also, the braincase is narrowing in a somewhat different way in each genus, which is well visible especially on the coronal suture. In Lophuromys and Arvicanthis, the braincase is also flattening, which is not the case with Stenocephalemys. Instead, the skull of Stenocephalemys gets more concave from the profile due to increasingly beveled shape of the rostrum. The occipital region is shortened in Lophuromys and Stenocephalemys but not in Arvicanthis. The zygomatic bone is getting wider with increasing elevation in all three genera, but trends in the overall shape of zygomatic arches are different. In Stenocephalemys they get wider in their maxillary section, and in Arvicanthis they shift dorsally and get tilted so they appear flat from the dorsal point of view. Finally, the zygomatic plate is higher in high-elevation species of all three genera, but in Lophuromys it is also wider and in Arvicanthis it is more curved.
Mandible shape differences associated with zonal gradation (Fig. 8 and S5) are found mostly on the coronoid and angular process, but each genus possesses a unique combination of features. The most conspicuous change in the shape of Lophuromys mandible is shallower transition from the coronoid to the condylar process, but the mandible is also getting deeper in its body. In Arvicanthis, the mandible is getting narrower and its angular process longer. In Stenocephalemys, the angular process gets shorter and the coronoid process lower, so the distance between their tips is also getting shorter.
The additional elevation-related shape changes, captured by PLS2, are shown in Figs. S6 (skull) and S7 (mandible) of the Supplementary Material 2. Here, the extreme associated with mid-elevation species is painted with yellow, whereas the opposite is in grey (for the ordination of species in the PLS1-2 space, see Fig. 4). In Lophuromys, the skull shape difference involves mostly the nasal bone, which is smaller in mid-elevation L. chercherensis and L. flavopunctatus and high-elevation L. simensis. The mandible shape PLS2 makes Lophuromys mid-elevation species distinctive with longer coronoid and wider angular process. In Arvicanthis, the mid-elevation A. saturatus is very close to high-elevation species in the skull shape (although note some difference in ventral view), but it is distinctive in its mandible shape which shows slightly longer coronoid and considerably smaller angular process. In Stenocephalemys, the mid-elevation species are distinctive in both skull and mandible shape. Their inter-orbital constrictions are narrower than predicted by PLS1, but their zygomatic arches retain shape of low-elevation species. Their mandibles have a bit longer coronoid process and they are wider in their posterior ramus.
Intra- and interspecific variation in skull size is shown in Fig. 9. In Arvicanthis and Lophuromys \(\Delta {\text{AIC}}\) was − 2.04 and − 0.18, respectively, i.e., the inclusion of elevation zone as a predictor did not improve model performance. In Stenocephalemys, on the contrary, \(\Delta {\text{AIC}}\) was 4.01 and Tukey tests revealed high-elevation species being different from low-elevation species (P < 0.0001) as well as from mid-elevation species (P = 0.0144), whereas the difference between low- and mid-elevation Stenocephalemys was not significant (P = 0.4103). More concretely, the high-elevation species are larger than those from lower elevation zones (Fig. 9). Mandible size is highly correlated (r = 0.97) with the skull size and the test results were also similar. In Arvicanthis the model with elevation zone was not supported (\(\Delta {\text{AIC}} = - 0.87\)), in Lophuromys its support was weak (\(\Delta {\text{AIC}} = 0.55\)) and in Stenocephalemys strong (\(\Delta {\text{AIC}} = 4.62\)). We performed Tukey tests for both genera with positive \(\Delta {\text{AIC}}\). In Lophuromys, only the difference between high- and low-elevation species reached marginal significance (P = 0.0423), while differences between high- and mid-elevation as well as mid- and low-elevation species were not significant (P = 0.1530 and P = 0.8271, respectively). In Stenocephalemys, the highly significant difference (P < 0.0001) was between high- and low-elevation species, whereas the other two differences were marginally non-significant (P = 0.0796 for high- vs. mid-elevation and P = 0.0604 for mid- vs. low-elevation species). For the visualization of intra- and interspecific variation in mandible size, see Fig. S8 in the Supplementary Material 2.
Intra- an interspecific variation in skull centroid size. Within each genus the species are ordered according to the elevation range of their occurrence, the dots are coloured according to the elevation zones as in Fig. 4
Discussion
As demonstrated in our study, species of the three rodent genera show a progressive shape differentiation along steep elevational gradients in Ethiopian highlands. In other words, an important component of interspecific variation exists, which differentiates high-elevation species from mid-elevation species as well as mid-elevation species from low-elevation ones. This trend was captured by PLS1 component apparent in both skull shape and mandible shape variation and although it does not account for the whole elevation-related differentiation, it still represents a prominent pattern of evolutionary change. In all three genera it shows mid-elevation species as intermediates, dissimilar to low-elevation as well as to high-elevation ones. Nevertheless, the mid-elevation species also possess some specific features—in all genera and both studied structures they are separated from the low- and high-elevation species due to presence of an additional (PLS2) trend. The PLS analysis identified some shape changes as universally shared, whereas other as genus-specific. This is in accord with the results of permutation tests, where significant interaction terms indicate the genera did not evolve identically.
Shared Patterns of Shape Differentiation
In all three genera, some regions of the skull are changing in the same or similar way with the increasing elevation: the interorbital constriction and the top of the braincase are getting narrower, zygomatic plate and the fossa adjacent to the infraorbital foramen are enlarged and the rostrum is prolonged. These shared changes have two general functional interpretations. First, in open habitats, it becomes increasingly important to watch for aerial predators (owls and birds of prey) which is assisted by the shift of eyes to the top of the head, so the field of view covers better the space above the animal (Fadda & Corti, 2000), possibly also due to better perception of depth. The shift is possible due to narrowing the skull between orbits, a change most pronounced in Stenocephalemys and Lophuromys (Fig. 4) but contained in all three PLS1 trends (Fig. 7). In external appearance, the upper position of eyes is best visible in S. albocaudatus, whose interorbital constriction is the narrowest one in absolute terms. The same morphological feature is in an extreme form present also in another Ethiopian high-elevation specialist, namely the giant root rat (Tachyoryctes macrocephalus) (Beolchini & Corti, 2004). This subterranean spalacid rodent adapted to life in Afroalpine moorlands by increasing its aboveground activity and hence the risk of predation, which is, in turn, partly alleviated by this morphological change.
Second, the structures involved in the shared changes are prominently associated with the masticatory apparatus (Baverstock et al., 2013), which suggests an effect of dietary adaptations. In all three genera the elevational trend (Figs. 7, 8) involved enlargement and consequently also anterior shift of the fossa adjacent to the infraorbital foramen, which is the insertion site of zygomatico-mandibularis muscle. The enlargement of this insertion site and so the widening of zygomatic arches in Stenocephalemys may reflect larger mass of the muscle which runs through the infraorbital foramen and the orbit. Anterior position of this insertion site was argued to provide strong pressure at the molars while allowing fine control at the incisors (Cox & Jeffery, 2015). A balance between the two actions can be modified differently in the studied genera, however, because the opposite insertion sites (mediodorsal surface of the zygomatic arch and then dorsolateral surface of the mandible) show different elevational trends. In Arvicanthis, the zygomatic arch was shifted upwards and tilted towards the midplane, in Lophuromys, this part of was wider and in Stenocephalemys, the mandible was slender in the attachment area. Differences in the shape of the coronoid process may reflect changes in size and function of the temporalis muscle, which is supposed to act as an elevator of the jaw and the retractor of the mandible. Finally, modification of the ventrolateral surface of the zygomatic arch (in Arvicanthis and Stenocephalemys) and disparate morphologies of the angular process of the mandible, may reflect changes in the working of deep masseter, a muscle supposed to provide major chewing power at the molars (Cox & Jeffery, 2015). A detailed study of diet and masticatory muscles would be necessary, however, for more conclusive interpretation of these differences. Nevertheless, the deeper body and stubbier coronoid process of Lophuromys mandible can suggest shift to herbivory and the slender ramus of high-elevation Stenocephalemys shift to insectivory if we compare to what is known for other rodents in terms of ecomorphology (cf. Michaux et al., 2007).
Components of Elevational Trends
So far, we discussed the main (PLS1) elevational trend. The additional (PLS2) trend either distinguishes mid-latitude species from being just an average of low- and high-latitude forms or it contributes to separation of all species from different zones, not only those at extremes of the main, PLS1, trend. In Stenocephalemys, PLS2 shows mid-elevation species to be much like high-elevation species in some features (inter-orbital constriction) but not others (shape of the zygomatic arch). In Arvicanthis, on the contrary, the mandible of mid-elevation A. saturatus resembles more the high-elevation forms but its angular process which is similar to low-elevation species. Finally, in Lophuromys, there is not a single elevational trend. L. melanonyx is markedly different from the rest if the genus (cf. Bekele & Corti, 1994) and it is only the combination of PLS1 and PLS2 which shows the differentiation also among the other species. Specific interpretation of these patterns would require better knowledge of other traits, especially the diet. They show, however, that mid-elevation species are not just intermediates of a single differentiation process.
Mixed Evidence for Size Differentiation
The size of skull and mandible is correlated with body mass (Bertrand et al., 2016a; b), which is predicted by Bergmann’s rule to be larger in species inhabiting cooler environment (Blackburn & Hawkins, 2004). The evidence for this pattern is mixed (Clauss et al., 2013; Feijó et al., 2019), however, and so are the results of our study. Stenocephalemys was the only genus with unequivocal support for high-elevation specialists being larger than the other species. There are at least two explanations for this observation. The larger size can either contribute to heat conservation in the cold, low-oxygen environment (Steudel et al., 1994), or it can be associated with shift towards low turnover population dynamics including less offspring, longer survival, and life span (Yoccoz & Mesnager, 1998). In any case, Stenocephalemys appears a good model to test these, mutually non-exclusive, hypotheses.
In Lophuromys and Arvicanthis, no significant difference was observed, but even here we can observe some tendency towards an increase of skull (and hence body) size in high elevations. The largest species of Lophuromys is L. melanonyx, arguably the most extreme high-elevation specialist in the genus. The species is most differentiated also in its skull shape and it is also the only one, which is found exclusively on the Afroalpine moorlands. The non-significance of size difference between high- and low-elevation Lophuromys is clearly due to L. simensis, which lives mostly in the Afroalpine zone, but can be found also in lower elevations (Bryja et al., 2019a). In Arvicanthis, the largest species is also high-elevation specialist, namely A. blicki, although it is less outlying in its genus than L. melanonyx among other Lophuromys. Possibly significant is the observation that A. blicki possess larger amount of brown adipose tissue than other Afroalpine rodents (Lövy et al., 2023). Increase in both body size and relative amount of brown adipose tissue can help an organism to survive in cold environment and so these traits can possibly compensate for each other. Data from other species of Arvicanthis are necessary, however, to test this hypothesis.
Evolution of Shape Differences
Published phylogenies of the three genera suggest the evolution of elevation-related traits had to be remarkably fast. In Arvicanthis, the basal divergence of the included species was dated to 0.8 Ma (Mikula et al., 2021) and the high-elevation species A. abyssinicus and A. blicki form a sister pair (Bryja et al., 2019b) whose closest relative is the mid-elevation A. saturatus (our unpublished data). It means their distinctive phenotype evolved on the scale of hundreds of thousands of years. The same applies to Stenocephalemys, whose basal divergence is also about 0.8 Ma old (Nicolas et al., 2021). Here, the phylogenetic relationships constrain the time scale of evolution even more because the closest relatives of the high-elevation species are low-elevation forest-dwellers. The most distinctive species, S. albocaudatus, split from S. ruppi 0.3 Ma ago and somewhat less differentiated S. zimai from S. albipes just about 0.1 Ma ago (Nicolas et al., 2021). We therefore observe occurrence of parallel evolution within this genus. In Lophuromys, the clade including inconspicuously differentiated L. simensis is ~ 0.5 Ma old, while the origin of highly distinctive L. melanonyx was put to ~ 1.1 Ma ago (Komarova et al., 2021). Here, the parallel evolution also occurred, although it did not come to comparably conspicuous end as in Stenocephalemys. The dates are better treated as tentative because we have no resolved nuclear phylogeny and no fossils for the genus, but as they are they also suggest high-elevation phenotypes to evolve on the timescale of the hundreds of thousands of years. There is a need for comprehensive, well-resolved time-calibrated phylogenies and additional Pleistocene fossils that would allow to estimate the tempo of evolution with more precision and accuracy and to infer the ancestral phenotype. Now, we can just assume it was not close to the high-elevation one, but otherwise our inferential power is limited.
Notably, the most extremely differentiated high-elevation species – L. melanonyx, A. blicki and S. albocaudatus – are found in the south-eastern block of Ethiopian highlands. The elevational zonation might be more ancient there. In means, the zonal biomes or their analogues would be better preserved throughout the Pleistocene climatic fluctuations so their specialists could survive unfavourable times in some refugia. This hypothesis could be directly tested via deep enough palaeoenvironmental records, but indirect support for it comes also from the presence of multiple well differentiated forms. In addition, it should be noted that there are other specialist species endemic to the area. For instance, Nikolaus’s African climbing-mouse (Megadendromus nikolausi) lives only in the ericaceous belt of Bale and Arsi Mts., while the giant root rat (Tachyoryctes macrocephalus) lives exclusively in their Afroalpine moorland. On the other hand, Mus imberbis was found in Afroalpine moorland of both south-eastern (Meheretu et al., 2015) and north-western block (Craig et al., 2020). Investigation of past demography of these nowadays isolated populations could also bring insight into persistence of zonal biomes across Ethiopia.
Skull Shape Evolution on Other Elevational Gradients
Similar patterns of skull shape differentiation can be expected also on other steep elevational gradients, where the same ecological zonation persists in a long-term, just shifting along the slope when climate changes. In Africa, such elevational zonation is developed on Virunga Mts. and Kilimanjaro. Small mammals of Virunga Mts. are yet to be thoroughly explored, but on Kilimanjaro, we do not see an analogy of the Ethiopian communities consisting of closely related zonal specialists (Stanley et al., 2014). Some observations suggest, however, that similar patterns could be found elsewhere in the world. In Papua New Guinea, for instance, elevational gradients span more than 4000 m of elevation, are correlated with diverse habitats from lowland alluvial forest to upper montane grasslands (Paijmans, 1976). They host rodent communities with closely related species often inhabiting different elevation zones (Roycroft et al., 2022). Elevation-related morphological changes were already reported in several species of Pseudohydromys (Helgen & Helgen, 2009). Also, the elevational gradients in Andes host a rich fauna of sigmodontine rodents (Parada et al., 2015), including genera with different elevation specialists (D’Elía et al., 2008; Steppan, 1998). When studied across South American rodent communities, some skull shape features were identified as typical for fauna of open and seasonal environments including those of the western slope of the Andes (Maestri et al., 2018).
Conclusion and Outlook
As far as we know this is the first study systematically comparing skull shape in closely related rodent species specialized to different zones of the same elevational gradients. In addition, we studied the differentiation in three genera that can be considered independent replicates of the same evolutionary process. In all of them, the elevation-related differentiation represented a large proportion of interspecific variation. The comparison further revealed some shape changes were shared across the genera, while in others the same structures were involved even though they changed differently. The similarity of non-sister specialists from the same genus suggests that in some cases the differentiation ran in parallel. It was also revealed that the differentiation can be fast (relative to species lifespan).
The phenotypic differentiation of closely related species or populations on elevational or other ecological gradients is presumably common in nature. If persistent over long time, such zonation is expected to support differentiation of whole communities with multiple species adapted to the same general conditions. The methodology proposed here helps to scrutinize evolutionary processes associated with such community level differentiation. It allows to distinguish universally shared phenotypic changes from a mosaic of species-specific adaptations and identify traits that are typically differentiated, although perhaps in different ways. If combined with phylogenetic information, it can identify instances of parallel evolution. When functional interpretation of the observed differences is available, it can tell apart convergence of form and function. In the context of our present study, the PLS approach could be used to test whether the same shape changes are present in rodents of other elevation gradients or whether the same evidence for parallel evolution will be found also in other vertebrates of Ethiopian highlands.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adams, D. C., Collyer, M. L., & Kaliontzopoulou, A. (2020). Geomorph: Software for geometric morphometric analyses. R package version 3.3.1. https://cran.r-project.org/package=geomorph
Adler, D., Murdoch, D., et al. (2020). rgl: 3D Visualization Using OpenGL. R package version 0.100.54. https://cran.r-project.org/package=rgl
Aghová, T., Kimura, Y., Bryja, J., Dobigny, G., Granjon, L., & Kergoat, G. J. (2018). Fossils know it best: Using a new set of fossil calibrations to improve the temporal phylogenetic framework of murid rodents (Rodentia: Muridae). Molecular Phylogenetics and Evolution, 128, 98–111. https://doi.org/10.1016/j.ympev.2018.07.017
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723. https://doi.org/10.1109/TAC.1974.1100705
Asefa, M., Cao, M., He, Y., Mekonnen, E., Song, X., & Yang, J. (2020). Ethiopian vegetation types, climate and topography. Plant Diversity, 42(4), 302–311. https://doi.org/10.1016/j.pld.2020.04.004
Baverstock, H., Jeffery, N. S., & Cobb, S. N. (2013). The morphology of the mouse masticatory musculature. Journal of Anatomy, 223(1), 46–60. https://doi.org/10.1111/joa.12059
Bekele, A., & Corti, M. (1994). Multivariate morphometrics of the Ethiopian populations of harsh-furred rat (Lophuromys, Mammalia, Rodentia). Journal of Zoology, 232(4), 675–689. https://doi.org/10.1111/j.1469-7998.1994.tb04621.x
Belay, G., & Mori, A. (2006). Intraspecific phylogeographic mitochondrial DNA (D-loop) variation of Gelada baboon, Theropithecus gelada, Ethiopia. Biochemical Systematics and Ecology, 34(7), 554–561. https://doi.org/10.1016/j.bse.2006.01.004
Beolchini, F., & Corti, M. (2004). The taxonomy of the genus Tachyoryctes: A geometric morphometric approach. Italian Journal of Zoology, 71(1), 35–43. https://doi.org/10.1080/11250000409356548
Bertrand, J. A., Delahaie, B., Bourgeois, Y. X., Duval, T., García-Jiménez, R., Cornuault, J., Bujol, B., Thébaud, C., & Milá, B. (2016a). The role of selection and historical factors in driving population differentiation along an elevational gradient in an island bird. Journal of Evolutionary Biology, 29(4), 824–836. https://doi.org/10.1111/jeb.12829
Bertrand, O. C., Schillaci, M. A., & Silcox, M. T. (2016b). Cranial dimensions as estimators of body mass and locomotor habits in extant and fossil rodents, Journal of Vertebrate Paleontology, 36(1), e1014905. https://doi.org/10.1080/02724634.2015.1014905
Blackburn, T. M., & Hawkins, B. A. (2004). Bergmann’s rule and the mammal fauna of northern North America. Ecology, 27(6), 715–724. https://doi.org/10.1111/j.0906-7590.2004.03999.x
Bookstein, F. L. (1997). Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Medical Image Analysis, 1(3), 225–243. https://doi.org/10.1016/S1361-8415(97)85012-8
Bookstein, F. L. (2018). A course in morphometrics for biologists: Geometry and statistics for studies of organismal form. Cambridge University Press. https://doi.org/10.1017/9781108120418
Bookstein, F. L. (1991). Morphometric tools for landmark data: Geometry and Biology. Cambridge University Press. https://doi.org/10.1017/CBO9780511573064
Bryja, J., Kostin, D., Meheretu, Y., Šumbera, R., Bryjová, A., Kasso, M., Mikula, O., & Lavrenchenko, L. A. (2018). Reticulate Pleistocene evolution of Ethiopian rodent genus along remarkable altitudinal gradient. Molecular Phylogenetics and Evolution, 118, 75–87. https://doi.org/10.1016/j.ympev.2017.09.020
Bryja, J., Colangelo, P., Lavrenchenko, L. A., Meheretu, Y., Šumbera, R., Bryjová, A., Verheyen, E., Leirs, H., & Castiglia, R. (2019b). Diversity and evolution of African grass rats (Muridae: Arvicanthis)—from radiation in East Africa to repeated colonization of northwestern and southeastern savannas. Journal of Zoological Systematics and Evolutionary Research, 57(4), 970–988. https://doi.org/10.1111/jzs.12290
Bryja, J., Meheretu, Y., Šumbera, R., & Lavrenchenko, L. A. (2019a). Annotated checklist, taxonomy and distribution of rodents in Ethiopia. Journal of Vertebrate Biology, 68(3), 117–213. https://doi.org/10.25225/fozo.030.2019
Cardini, A., O’Higgins, P., & Rohlf, F. J. (2019). Seeing distinct groups where there are none: Spurious patterns from between-group PCA. Evolutionary Biology, 46(4), 303–316. https://doi.org/10.1007/s11692-019-09487-5
Cheriet, M., Said, J. N., & Suen, C. Y. (1998). A recursive thresholding technique for image segmentation. IEEE Transactions on Image Processing, 7(6), 918–921. https://doi.org/10.1109/83.679444
Clauss, M., Dittmann, M. T., Müller, D. W., Meloro, C., & Codron, D. (2013). Bergmann′ s rule in mammals: A cross-species interspecific pattern. Oikos, 122(10), 1465–1472. https://doi.org/10.1111/j.1600-0706.2013.00463.x
Couvreur, T. L., Dauby, G., Blach-Overgaard, A., Deblauwe, V., Dessein, S., Droissart, V., Hardy, O. J., Harris, D. J., Janssen, S. B., Ley, A. C., Mackinder, B. A., Sonké, B., Sosef, M. S. M., Stévart, T., Svenning, JCh., Wieringa, J. J., Faye, A., Missoup, A. D., Tolley, K. A., … Sepulchre, P. (2021). Tectonics, climate and the diversification of the tropical African terrestrial flora and fauna. Biological Reviews, 96(1), 16–51. https://doi.org/10.1111/brv.12644
Cox, P. G., & Jeffery, N. (2015). The muscles of mastication in rodents and the function of the medial pterygoid. In P. Cox, & L. Hautier (Eds.) Evolution of the rodents: Advances in phylogeny, functional morphology and development (pp. 350–372). Cambridge University Press. https://doi.org/10.1017/CBO9781107360150.014
Craig, E. W., Stanley, W. T., Kerbis Peterhans, J. C., Bryja, J., & Meheretu, Y. (2020). Small terrestrial mammal distributions in Simien Mountains National Park, Ethiopia: A reassessment after 88 years. Journal of Mammalogy, 101(3), 634–647. https://doi.org/10.1093/jmammal/gyaa040
D’Elía, G., Pardiñas, U. F. J., Jayat, J. P., & Salazar-Bravo, J. (2008). Systematics of Necromys (Rodentia, Cricetidae, Sigmodontinae): Species limits and groups, with comments on historical biogeography. Journal of Mammalogy, 89(3), 778–790. https://doi.org/10.1644/07-MAMM-A-246R1.1
Dryden, I. L., & Mardia, K. V. (2016). Statistical shape analysis: with applications in R (vol. 995). Wiley. https://doi.org/10.1002/9781119072492
Ehrich, D., Gaudeul, M., Assefa, A., Koch, M. A., Mummenhoff, K., Nemomissa, S., Consortium, I., & Brochmann, C. (2007). Genetic consequences of Pleistocene range shifts: contrast between the Arctic, the Alps and the East African mountains. Molecular Ecology, 16(12), 2542–2559. https://doi.org/10.1111/j.1365-294X.2007.03299.x
Evans, B. J., Bliss, S. M., Mendel, S. A., & Tinsley, R. C. (2011). The Rift Valley is a major barrier to dispersal of African clawed frogs (Xenopus) in Ethiopia. Molecular Ecology, 20(20), 4216–4230. https://doi.org/10.1111/j.1365-294X.2011.05262.x
Fadda, C., & Corti, M. (2000). Three dimensional geometric morphometric study of the Ethiopian Myomys-Stenocephalemys complex (Murinae, Rodentia). Hystrix, the Italian Journal of Mammalogy, 10(2), 131–143. https://doi.org/10.4404/hystrix-11.1-4141
Fadda, C., & Corti, M. (2001). Three-dimensional geometric morphometrics of Arvicanthis: Implications for systematics and taxonomy. Journal of Zoological Systematics and Evolutionary Research, 39(4), 235–245. https://doi.org/10.1046/j.1439-0469.2001.00169.x
Feijó, A., Wen, Z., Cheng, J., Ge, D., Xia, L., & Yang, Q. (2019). Divergent selection along elevational gradients promotes genetic and phenotypic disparities among small mammal populations. Ecology and Evolution, 9(12), 7080–7095. https://doi.org/10.1002/ece3.5273
Fjeldså, J., Johansson, U. S., Lokugalappatti, L. S., & Bowie, R. C. (2007). Diversification of African greenbuls in space and time: Linking ecological and historical processes. Journal of Ornithology, 148(2), 359–367. https://doi.org/10.1007/s10336-007-0179-4
Freilich, X., Tollis, M., & Boissinot, S. (2014). Hiding in the highlands: Evolution of a frog species complex of the genus Ptychadena in the Ethiopian highlands. Molecular Phylogenetics and Evolution, 71, 157–169. https://doi.org/10.1016/j.ympev.2013.11.015
Friis, I. B., Demissew, S., & van Breugel, P. (2010). Atlas of the potential vegetation of Ethiopia. Det Kongelige Danske Videnskabernes Selskab. https://doi.org/10.1017/S0960428611000448
Gottelli, D., Marino, J., Sillero-Zubiri, C., & Funk, S. M. (2004). The effect of the last glacial age on speciation and population genetic structure of the endangered Ethiopian wolf (Canis simensis). Molecular Ecology, 13(8), 2275–2286. https://doi.org/10.1111/j.1365-294X.2004.02226.x
Gower, J. C. (1975). Generalized procrustes analysis. Psychometrika, 40, 33–51. https://doi.org/10.1007/BF02291478
Graham, C. H., Parra, M., Mora, A., & Higuera, C. (2018). The interplay between geological history and ecology in mountains. In C. Hoorn, A. Perrigo, & A. Antonelli (Eds.), Mountains, climate and biodiversity (pp. 231–243). Wiley.
Gunz, P., Mitteroecker, P., & Bookstein, F. L. (2005). Semilandmarks in three dimensions. In D. E. Slice (Ed.), Modern morphometrics in physical anthropology. Developments in Primatology: Progress and Prospects. (pp. 73–98). Springer. https://doi.org/10.1007/0-387-27614-9_3
Hastie, T., Tibshirani, R., & Friedman, J. (2001). The elements of statistical learning. Springer. https://doi.org/10.1007/978-0-387-84858-7
Helgen, K. M., & Helgen, L. E. (2009). Biodiversity and biogeography of the moss-mice of New Guinea: A taxonomic revision of Pseudohydromys (Muridae: Murinae). Bulletin of the American Museum of Natural History, 331, 230–313. https://doi.org/10.1206/582-8.1
Hothorn, T., Bretz, F., & Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical Journal: Journal of Mathematical Methods in Biosciences, 50(3), 346–363. https://doi.org/10.1002/bimj.200810425
Kebede, M., Ehrich, D., Taberlet, P., Nemomissa, S., & Brochmann, C. (2007). Phylogeography and conservation genetics of a giant lobelia (Lobelia giberroa) in Ethiopian and Tropical East African mountains. Molecular Ecology, 16(6), 1233–1243. https://doi.org/10.1111/j.1365-294X.2007.03232.x
Keller, I., Alexander, J. M., Holderegger, R., & Edwards, P. J. (2013). Widespread phenotypic and genetic divergence along altitudinal gradients in animals. Journal of Evolutionary Biology, 26(12), 2527–2543. https://doi.org/10.1111/jeb.12255
Klingenberg, C. P., Barluenga, M., & Meyer, A. (2002). Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution, 56(10), 1909–1920. https://doi.org/10.1111/j.0014-3820.2002.tb00117.x
Komarova, V. A., Kostin, D. S., Bryja, J., Mikula, O., Bryjová, A., Čížková, D., Šumbera, R., Meheretu, Y., & Lavrenchenko, L. A. (2021). Complex reticulate evolution of speckled brush-furred rats (Lophuromys) in the Ethiopian centre of endemism. Molecular Ecology, 30(10), 2349–2365. https://doi.org/10.1111/mec.15891
Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends in Ecology & Evolution, 22(11), 569–574. https://doi.org/10.1016/j.tree.2007.09.006
Lamb, A. L., Leng, M. J., Mohammed, M. U., & Lamb, H. F. (2004). Holocene climate and vegetation change in the Main Ethiopian Rift Valley, inferred from the composition (C/N and d13C) of lacustrine organic matter. Quaternary Science Reviews, 23(7–8), 881–891. https://doi.org/10.1016/j.quascirev.2003.06.010
Lavrenchenko, L. A., Verheyen, W. N., Verheyen, E. K., Hulselmans, J., & Leirs, H. (2007). Morphometric and genetic study of Ethiopian Lophuromys flavopunctatus Thomas, 1888 species complex with description of three new 70-chromosomal species (Muridae, Rodentia). Bulletin De L’institut Royal Des Sciences Naturelles De Belgique, 77, 77–117.
Lövy, M., Boratyński, Z., Okrouhlík, J., Svačinová, L., Meheretu, Y., & Šumbera, R. (2023). Alternative highland adaptations: Organ masses and fur insulation in Afroalpine rodents. Journal of Zoology, 319(2), 119–130. https://doi.org/10.1111/jzo.13028
Maestri, R., Monteiro, L. R., Fornel, R., Ochotorena de Freitas, T. R., & Patterson, B. D. (2018). Geometric morphometrics meets metacommunity ecology: Environment and lineage distribution affects spatial variation in shape. Ecography, 41(1), 90–100. https://doi.org/10.1111/ecog.03001
Marshall, M. H., Lamb, H. F., Davies, S. J., Leng, M. J., Kubsa, Z., Umer, M., & Bryant, C. (2009). Climatic change in northern Ethiopia during the past 17,000 years: A diatom and stable isotope record from Lake Ashenge. Palaeogeography, Palaeoclimatology, Palaeoecology, 279(1–2), 114–127. https://doi.org/10.1016/j.palaeo.2009.05.003
Meheretu, Y., Šumbera, R., & Bryja, J. (2015). Enigmatic Ethiopian endemic rodent Muriculus imberbis (Rüppell 1842) represents a separate lineage within genus Mus. Mammalia, 79(1), 15–23. https://doi.org/10.1515/mammalia-2013-0119
Michaux, J., Chevret, P., & Renaud, S. (2007). Morphological diversity of Old World rats and mice (Rodentia, Muridae) mandible in relation with phylogeny and adaptation. Journal of Zoological Systematics and Evolutionary Research, 45(3), 263–279. https://doi.org/10.1111/j.1439-0469.2006.00390.x
Mikula, O., Nicolas, V., Šumbera, R., Konečný, A., Denys, C., Verheyen, E., Bryjová, A., Lemmon, A. R., Lemmon, E. M., & Bryja, J. (2021). Nuclear phylogenomics, but not mitogenomics, resolves the most successful Late Miocene radiation of African mammals (Rodentia: Muridae: Arvicanthini). Molecular Phylogenetics and Evolution, 157, 107069. https://doi.org/10.1016/j.ympev.2021.107069
Mizerovská, D., Mikula, O., Meheretu, Y., Bartáková, V., Bryjová, A., Kostin, D. S., Šumbera, R., Lavrenchenko, L. A., & Bryja, J. (2020). Integrative taxonomic revision of the Ethiopian endemic rodent genus Stenocephalemys (Muridae: Murinae: Praomyini) with the description of two new species. Journal of Vertebrate Biology, 69(2), 20031. https://doi.org/10.25225/jvb.20031
Mosbrugger, V., Favre, A., Muellner-Riehl, A. N., Päckert, M., & Mulch, A. (2018). Cenozoic evolution of geo-biodiversity in the Tibeto-Himalayan region. In C. Hoorn, A. Perrigo, & A. Antonelli (Eds.), Mountains, climate and biodiversity (pp. 429–448). Wiley.
Nicolas, V., Mikula, O., Lavrenchenko, L. A., Šumbera, R., Bartáková, V., Bryjová, A., Meheretu, Y., Verheyen, E., Missoup, A. D., Lemmon, A. R., Lemmon, E. M., & Bryja, J. (2021). Phylogenomics of African radiation of Praomyini (Muridae: Murinae) rodents: First fully resolved phylogeny, evolutionary history and delimitation of extant genera. Molecular Phylogenetics and Evolution, 163, 107263. https://doi.org/10.1016/j.ympev.2021.107263
Ohsawa, T., & Ide, Y. (2008). Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Global Ecology and Biogeography, 17(2), 152–163. https://doi.org/10.1111/j.1466-8238.2007.00357.x
Oksanen, J., Simpson, G., Blanchet, F., Kindt, R., Legendre, P., Minchin, P., O’Hara, R., Solymos, P., Stevens, M., Szoecs, E., Wagner, H., Barbour, M., Bedward, M., Bolker, B., Borcard, D., Carvalho, G., Chirico, M., De, Caceres, M., Durand, S., Evangelista, H., FitzJohn, R., Friendly, M., Furneaux, B., Hannigan, G., Hill, M., Lahti, L., McGlinn, D., Ouellette, M., Ribeiro, Cunha, E., Smith, T., Stier, A., Ter Braak, C., & Weedon, J. (2022). vegan: Community Ecology Package. R package version 2.6-4. https://CRAN.R-project.org/package=vegan
Paijmans, K. (1976). New Guinea vegetation. Australian National University Press.
Parada, A., D’Elía, G., & Palma, R. E. (2015). The influence of ecological and geographical context in the radiation of neotropical sigmodontine rodents. BMC Evolutionary Biology, 15, 172. https://doi.org/10.1186/s12862-015-0440-z
Pinheiro, J., Bates, D., DebRoy, S., & Sarkar, D. (2020). nlme: Linear and nonlinear mixed effects models. R package version 3.1-148. https://cran.r-project.org/package=nlme
R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org
Rohlf, F. J. (1999). Shape statistics: Procrustes superimpositions and tangent spaces. Journal of Classification, 16(2), 197–223. https://doi.org/10.1007/s003579900054
Rohlf, F. J., & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology, 39(1), 40–59. https://doi.org/10.2307/2992207
Rosipal, R., & Krämer, N. (2005). Overview and recent advances in partial least squares. In C. Saunders, M. Grobelnik, S. Gunn, & J. Shawe-Taylor (Eds.), International statistical and pptimization perspectives workshop “Subspace, latent structure and feature selection” (pp. 34–51). Springer. https://doi.org/10.1007/11752790_2
Roycroft, E., Fabre, P.-H., MacDonald, A. J., Moritz, C., Moussalli, A., & Rowe, K. C. (2022). New Guinea uplift opens ecological opportunity across a continent. Current Biology, 32(19), 4215-4224.e3. https://doi.org/10.1016/j.cub.2022.08.021
Sarkar, D., & Andrews, F. (2019). latticeExtra: Extra graphical utilities based on lattice. R package version 0.6-30. https://cran.r-project.org/package=latticeExtra.
Sarkar, D. (2008). Lattice: multivariate data visualization with R. Springer. https://doi.org/10.1007/978-0-387-75969-2
Schlager, S. (2017). Morpho and Rvcg–Shape Analysis in R: R-Packages for geometric morphometrics, shape analysis and surface manipulations. In G. Zheng, S. Li, & G. Szekely (Eds.), Statistical shape and deformation analysis (pp. 217–256). Academic Press. https://doi.org/10.1016/B978-0-12-810493-4.00011-0
Stanley, W. T., Rogers, M. A., Kihaule, P. M., & Munissi, M. J. (2014). Elevational distribution and ecology of small mammals on Africa’s highest mountain. PLoS One, 9(11), e109904. https://doi.org/10.1371/journal.pone.0109904
Steppan, S. J. (1998). Phylogenetic relationships and species limits within Phyllotis (Rodentia: Sigmodontinae): Concordance between MTDNA sequence and morphology. Journal of Mammalogy, 79(2), 573–593. https://doi.org/10.2307/1382988
Steudel, K. L., Porter, W. P., & Sher, D. (1994). The biophysics of Bergmann’s rule: A comparison of the effects of pelage and body size variation on metabolic rate. Canadian Journal of Zoology, 72(1), 70–77. https://doi.org/10.1139/z94-010
Šumbera, R., Krásová, J., Lavrenchenko, L. A., Mengistu, S., Bekele, A., Mikula, O., & Bryja, J. (2018). Ethiopian highlands as a cradle of the African fossorial root-rats (genus Tachyoryctes), the genetic evidence. Molecular Phylogenetics and Evolution, 126, 105–115. https://doi.org/10.1016/j.ympev.2018.04.003
Suzuki, T., Suzuki, N., & Tojo, K. (2019). Parallel evolution of an alpine type ecomorph in a scorpionfly: Independent adaptation to high-altitude environments in multiple mountain locations. Molecular Ecology, 28(13), 3225–3240. https://doi.org/10.1111/mec.15119
Taylor, P. J., Lavrenchenko, L. A., Carleton, M. D., Verheyen, E., Bennett, N. C., Oosthuizen, C. J., & Maree, S. (2011). Specific limits and emerging diversity patterns in East African populations of laminate-toothed rats, genus Otomys (Muridae: Murinae: Otomyini): Revision of the Otomys typus complex. Zootaxa, 3024, 1–66.
Tukey, J. W. (1949). Comparing individual means in the analysis of variance. Biometrics, 5(2), 99–114. https://doi.org/10.2307/3001913
van Breugel, P., Kindt, R., Lillesø, J.P.B., Bingham, M., Demissew, S., Dudley, C., Friis, I., Gachathi, F., Kalema, J., Mbago, F., Moshi, H.N., Mulumba, J., Namaganda, M., Ndangalasi, H.J., Ruffo, C.K., Védaste, M., Jamnadass, R., & Graudal, L. (2015). Potential Natural Vegetation Map of Eastern Africa (Burundi, Ethiopia, Kenya, Malawi, Rwanda, Tanzania, Uganda and Zambia). Version 2.0. Forest & Landscape Denmark and World Agroforestry Centre (ICRAF). https://vegetationmap4africa.org
Wold, H. (1975). Path models with latent variables: The NIPALS approach. In H. M. Blalock, A. Aganbegian, F. M. Borodkin, R. Boudon, & V. Capecchi (Eds.), Quantitative sociology (pp. 307–357). Academic Press. https://doi.org/10.1016/B978-0-12-103950-9.50017-4
Yoccoz, N. G., & Mesnager, S. (1998). Are alpine bank voles larger and more sexually dimorphic because adults survive better? Oikos, 82(1), 85–98. https://doi.org/10.2307/3546919f
Acknowledgements
For help in the field and with logistics, we acknowledge Y. Meheretu, R. Šumbera, V. Mazoch, T. Aghová, A. Ribas, J. Šklíba, M. Lövy, D. S. Kostin, A. A. Martynov, A. R. Gromov, D. Yu. Alexandrov, M. Kasso, and all local collaborators.
Funding
Open access publishing supported by the National Technical Library in Prague. This study was supported by the Czech Science Foundation (projects no. 18-17398S and 20-07091J) and the Russian Foundation for Basic Research (project no. 19-54-26003).
Author information
Authors and Affiliations
Contributions
DM, JB, OM conceived the study, JB provided funding, DM, LAL, JB, OM sampled material in the field, DM, FŠ, JP collected morphometric data, DM, JC, OM analysed morphometric data, DM, JB, OM drafted the manuscript. All authors contributed to the editing of the manuscript, gave final approval for publication, and agreed to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research Permissions
All fieldwork complied with legal regulations in Ethiopia and sampling was carried out with the permission of the Ethiopian Wildlife Conservation Authority and the Oromia Forest and Wildlife Enterprise.
Supplementary Information
Below is the link to the electronic supplementary material.
11692_2023_9608_MOESM1_ESM.xlsx
Supplementary file1 (XLSX 20 kb) Supplementary Material 1: List of the specimens analysed, including their taxonomic classification, georeferencing information and collection data
11692_2023_9608_MOESM2_ESM.docx
Supplementary file2 (DOCX 1337 kb) Supplementary Material 2: Supplementary figures showing geographic origin of individuals analysed in this study and distribution of major vegetation types (Fig. S1), asymmetric variation of skull and mandible (Fig. S2), results of PLS analysis based on fixed landmarks only (Fig. S3), comparison of elevation-related shape changes of skull and mandible (Figs. S4, S5), shape differences accounted for by PLS2 (Figs. S6, S7) and mandible size variation (Fig. S8). Supplementary table with anatomic definitions of the fixed landmarks (Table S1)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mizerovská, D., Špoutil, F., Claude, J. et al. Parallel Evolution of Skull Form in Three Rodent Genera Inhabiting Steep Elevational Gradients of Ethiopian Highlands. Evol Biol 50, 332–349 (2023). https://doi.org/10.1007/s11692-023-09608-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-023-09608-1