Abstract
Purpose
Derogenes ruber Lühe, 1900, the type-species of the genus Derogenes Lühe, 1900, is a poorly known derogenid digenean. The original description of this species was not illustrated and aspects of the morphology of the parasite from the type-host remain scarce. Available records of this species were brief and/or lacked illustrations and were based on morphology alone. Additionally, molecular data for Derogenes spp. are warranted to untangle species complexes as they provide a better assessment of interspecific genetic divergence.
Methods
Derogenes ruber is redescribed based on newly collected specimens from the gall bladder of its type-host Chelidonichthys lastoviza (Bonnaterre, 1788) collected in the Western Mediterranean off the Algerian coast during 2017–2019 and molecular data are provided using a partial fragment of the nuclear 28S ribosomal RNA gene (28S rRNA), the internal transcribed spacer 2 (ITS2) and a fragment of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene.
Results
We herein provide a detailed illustrated redescription and morphometric data of D. ruber from its type-host C. lastoviza. We report a new geographical record (off Algeria) for it. Derogenes ruber is also genetically characterised for the first time. Species/lineages of Derogenes were recovered in five strongly supported reciprocally monophyletic clades: (i) D. ruber from C. lastoviza off Algeria; (ii) D. lacustris from Galaxias maculatus (Jenyns) off Argentina; (iii) Lineage “D. varicus DV1” (D. varicus sensu stricto) from fish hosts in the White and Barents seas and the North Sea; (iv) Lineage “D. varicus DV2” from mollusc hosts in the White Sea; and (v) Lineage “D. varicus DV3” from Eumicrotremus fedorovi Mandrytsa. in the Pacific Ocean. Hence, comparison of the newly generated sequences with other available data for Derogenes species supports the distinction of D. ruber confirming its taxonomic status and helping assess interspecific variation. Comparison of D. ruber with the closely related species Derogenes latus revealed overlaps in morphometric data and the validity of the latter species is questioned.
Conclusion
The combination of morphological and molecular data provided for D. ruber provides a firm foundation for further investigations of Derogenes spp. Although we do describe herein material of D. ruber from the type-host, given that the occurrence of a single Derogenes species in various hosts has been challenged by molecular data, and both D. lacustris and D. varicus sensu stricto had been genetically proven to occur in various hosts, D. ruber and D. latus may be indeed synonymous. Additional sequencing effort on Derogenes spp. will strengthen systematic comparative studies and evolutionary relationships within the Derogenidae in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Derogenids are hemiuroid digenean gut parasites, occurring in fishes. Throughout most of their taxonomic history, they were accommodated within a broad concept of the family Hemiuridae Looss, 1899 [1]. The Derogenidae Nicoll, 1910 was first used at full family rank by Dollfus [2] but was initially erected at the subfamily level by Nicoll [3] as the Derogeninae Nicoll, 1910 (referred to as the Derogeninae Dollfus, 1950 by Skrjabin and Guschanskaja [4]). The latter authors included the subfamily within the Halipegidae Poche, 1926, but the Derogenidae has priority [1].
Gibson and Bray [5] established the initial foundational classification of the Derogenidae, offering identification keys for its subfamilies and genera. Within this context, Gibson [1] acknowledged the presence of three subfamilies: Derogeninae Nicoll, 1910, Halipeginae Poche, 1926, and Gonocercinae Skrjabin & Guschanskaja, 1955. At present, the Derogenidae comprises only two subfamilies: Halipeginae Poche, 1926, and Derogeninae [1]. This adjustment in classification occurred due to a molecular study conducted by Sokolov et al. [6], who elevated the Gonocercinae to the status of a full family.
Five valid genera are included in the Derogeninae: Derogenes Lühe, 1900, Gonocercella Manter, 1940 [7], Leurodera Linton, 1910, Progonus Looss, 1899, and Derogenoides Nicoll, 1913 [1, 8].
Records of derogenine derogenids in the Mediterranean are rare [9]. Thus, previous records of Derogenes spp. in this region include D. adriaticus Nikolaeva, 1966, D. crassus Manter, 1934, D. fuhrmanni Mola, 1912, D. latus Janiszewska, 1953, D. minor Looss, 1901, D. ruber Lühe, 1900, and D. varicus (Müller, 1784) [10, 11]. However, most of the records lack morphological information justifying correct species identification and making the assessment of host–parasite associations difficult if not impossible. For example, only in the Mediterranean, D. varicus has been reported in 15 hosts of 13 unrelated fish families [11] indicating that this “generalist” species may represent a species complex. This has been suggested by Bray et al. [12] and Køie [13], and a recent study based on multigene sequence data supported this suggestion by providing evidence for the existence of four genetic lineages of D. varicus [14, 15].
Derogenes ruber, the type-species of the genus was less frequently encountered and reported. The type-material of D. ruber was described by Lühe [16] from the gall-bladder of the streaked gurnard Trigla lineata Gmelin, 1789 (a junior synonym of Chelidonichthys lastoviza (Bonnaterre, 1788)) off Rovinj, Croatia, Adriatic Sea. This trematode is known from the short original description that lacked illustrations, and a subsequent general illustration, based on a record and identification from a different host, the piper gurnard Trigla lyra L. from a close locality, off Split, Croatia [17]. Bouguerche et al. [15] redescribed this species based only on two specimens found in Arthur Looss’s collection and did not thus provide any molecular data. Other reports of this derogenid are from the North-East Atlantic (off Azores, Canary and Cape Verde Islands [18], and off Spain [19].
During parasitological surveys of helminths of fishes from off the southern coasts of the Western Mediterranean off Algeria, we collected representatives of D. ruber from the gall bladder of its type-host, C. lastoviza. The aim of the present study is to provide a formal redescription of D. ruber and to characterise the species genetically based on partial 28S ribosomal RNA gene (28S rRNA), internal transcribed spacer ITS2, and a fragment of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene sequences.
Materials and Methods
Collection and Sampling of Fish
A total of 168 specimens of C. lastoviza were collected during 2017–2019, from local fishermen immediately after capture in different regions off the Algerian coast: Ghazaouet (35° 06′ 0′′ N, 1° 51′ 0′′ W), Cherchell (36° 36′ 31′′ N, 2° 11′ 50′′ E), Bouharoune (36° 37′ 24′′ N, 2° 39′ 17′′ E), Alger (36° 45′ 8′′ N, 3° 2′ 31′′ E), Bordj el Bahri (36° 47′ 26′′ N, 3° 14′ 59′′ E), Ain Taya (36° 47′ 30′′ N, 3° 17′ 20′′ E), Reghaia (36° 43′ 60′′ N, 3° 21′ 0′′ E), Cap Djinet (36° 52′ 37′′ N, 3° 43′ 23′′ E), and Dellys (36° 54′ 48′′ N, 3° 54′ 51′′ E). Fish specimens were kept on ice and transferred immediately to the laboratory, identified using the key [20, 21], and examined on the day of purchase. Viscera were placed in separate Petri dishes containing seawater and observed under a Zeiss microscope for the presence of digeneans.
Morphological Methods
Live digeneans were killed and fixed in near-boiling water. Specimens for morphological analysis were fixed under cover-glass pressure in Bouin’s fluid [10], then preserved in 70% ethanol, stained with acetic carmine, dehydrated through a graded alcohol series, cleared in clove oil, and mounted in Canada balsam as permanent mounts. Five specimens were preserved immediately in 96% ethanol for molecular characterisation and were processed as hologenophores (sensu Pleijel et al. [22]).
Permanent mounts of the hologenophores, consisting of 2/3 of the body (posterior third excised and used for sequencing), stained and mounted in Canada balsam. Drawings were made using a Zeiss microscope (Université des Sciences et de la Technologie Houari Boumediene, USTHB) and a Nikon Eclipse i80 microscope with DIC (differential interference contrast) (Swedish Museum of Natural History, SMNH) equipped with a drawing tube, and scanned and redrawn with Adobe Illustrator 2023, version 28.0.
Measurements are in given in micrometres and presented as the range followed by the mean in parentheses. Voucher material was deposited at the Swedish Museum of Natural History (SMNH), Stockholm, Sweden under accession numbers SMNH 218781–SMNH 218 805.
Molecular Methods
Genomic DNA was extracted from a total of five hologenophores, and genetic sequence data were generated for three genetic markers: a partial region of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1), the second internal transcribed spacer region (ITS2 rDNA), and the large (28S) ribosomal RNA gene. A small fragment of each hologenophore (posterior third) was placed in a 1.5 ml microcentrifuge tube containing 20 μL buffer ATL (Qiagen, Hilden, Germany). For extraction of genomic DNA (gDNA), 20 μL buffer ATL and 20 μL proteinase K were added to each sample, followed by vortexing and incubation in an incubating microplate shaker at 56 °C and 300 rpm overnight. The lysed samples were processed to obtain gDNA following the manufacturer’s instructions for gDNA extraction using the Qiagen QiAmp DNA Microkit. Polymerase chain reaction (PCR) amplification was performed in 25 µl reaction mix using Illustra Hot Start Mix RTG (0.2 µl) reaction kit (GE Healthcare Life Sciences, Uppsala, Sweden). The reaction mix consisted of 1 µl (0.4 µM) of each primer, 2 µl template DNA, and 21 µl nuclease-free water. The primer set JB3 (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and COI R-Trema (5′-CAA CAA ATC ATG ATG CAA AAG G-3′) were used to amplify a fragment the cox1 gene [23]. The thermocycling profile consisted of an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 45 °C for 30 s, and extension at 72 °C for 1 min, with a final extension step at 72 °C for 10 min [14]. Primers, amplification, and sequencing protocols for the 28S rDNA region followed Pérez-Ponce de León et al. [24] and García-Varela and Nadler (2005) [25]. The thermocycling profile consisted of an initial denaturation step at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 60 s, annealing at 54 °C for 60 s, and extension at 72 °C for 1 min, with a final extension step at 72 °C for 7 min. ITS2 rDNA spacer was amplified using the primers 3S [26] and ITS2.2 [27] and the following thermocycling profile: an initial denaturation step at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 54 °C for 1 min, and extension at 72 °C for 1 min, with a final extension step at 72 °C for 7 min. PCR products were purified (Ampure XP Kit, Beckman Coulter, Indianapolis, USA) and sequenced in both directions on a 3730 l DNA Analyzer 96-capillary sequencer (Applied Biosystems, Foster City, CA, USA). We used CodonCode Aligner version 3.7.1 software (Codon Code Corporation, Dedham, MA, USA) to edit sequences and compared them to the GenBank database content using BLAST. The newly generated sequences are deposited in the GenBank database under the accession numbers OQ919798-OQ919804, OQ919806, OR245546, and OR245386.
Phylogenetic analyses were performed using the newly generated sequences of D. ruber and those for Derogenidae species available in GenBank (Table 1). Alignments for each gene region were constructed in AliView [28] and trimmed to the length of the shortest sequence. Nucleotide substitution models for phylogenetic analyses using the maximum-likelihood method were estimated using MEGA11 [29]. The best-fit models selected were the Kimura 2-parameter model with gamma distributed amongst-site rate variation (K2 + G) for the 28S rDNA alignment, Kimura 2-parameter (K2) model for the ITS2 alignment, and Tamura-Nei model (TN93) with estimates of invariant sites and gamma distributed amongst-site rate variation (HKY + I + G) for cox1. All trees were constructed in MEGA11, with 500 replications. Genetic distances [uncorrected p-distance model (Kimura 1980)] were computed with MEGA11.
Results
Family Derogenidae Nicoll, 1910
Subfamily Derogeninae Nicoll, 1910
Genus Derogenes Lühe, 1900
Derogenes ruber Lühe, 1900 (Fig. 1 A-E)
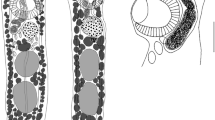
Derogenes ruber from Chelidonichthys lastoviza. A Hologenophore, ventral view, SMNH 218782. B Whole-body, ventral view, SMNH 218789. C Posterior extremity showing ends of caeca. D Egg, SMNH 218789. E Anterior extremity showing details of terminal genitalia, SMNH 218789. Abbreviations: C.: caecum; E.: egg; G.a.: genital atrium; G.p.: genital pore; M.d.: male duct; Ph.: pharynx; P.P.C.: prostatic cells; S.: sphincter; S.s.: sinus-sac; S.o.: sinus-organ; S.v.: seminal vesicle; U.: uterus
Type-host: Streaked gurnard Chelidonichthys lastoviza (syn. Trigla lineata) [16].
Other reported host: Piper gurnard Trigla lyra [17].
Type-locality: Off Rovinj, Croatia, Adriatic Sea [16].
Other localities: Off Split, Croatia, Adriatic Sea [17]; off Azores, Canary and Cape Verde islands [18] and off Spain [19], North-East Atlantic; Off Trieste, Italy, Western Mediterranean [15]; off Algeria, Western Mediterranean, present study.
Site in host: Gall bladder.
Other sites in host: Intestine [15].
Voucher material: A total of 25 voucher specimens are deposited in the collections of the Swedish Museum of Natural History, Stockholm (SMNH 218781- SMNH 218805) including 5 hologenophores (SMNH 218785, GenBank OR245386, OQ919806, OQ919799; SMNH 218786, GenBank OR245546, OQ919798; SMNH 218782, GenBank OQ919801, OQ919800; SMNH 218785, GenBank OQ919804; SMNH OQ919802, OQ919803).
Redescription
[Based on 20 specimens mounted in toto and 5 hologenophores, metrical data are provided in Table 2.] Body stout, fusiform (Fig. 1A, B), widest at ventral sucker level. Tegument smooth. Pre-oral lobe present. Oral and ventral suckers well developed; oral sucker ventro-subterminal, subglobular-to-globular, wider than long; ventral sucker larger than oral sucker, spherical, located in posterior half of body. Forebody somewhat longer than hindbody. Prepharynx absent. Pharynx well developed, subglobular, muscular. Oesophagus short, barely visible, opening posteriorly via sphincter (Fig. 1E) to join intestinal bifurcation in anterior half of forebody, immediately posterior to pharynx. ‘Drüsenmagen’ not observed. Caeca broad, thick walled, extending into hindbody, reaching beyond gonads, terminating close to posterior extremity (Fig. 1C). Termination of caeca often obscured by eggs.
Testes two, entire, rounded, symmetrical, pre-ovarian, posterior to ventral sucker and separated by uterine coils. Seminal vesicle external, tubular, thin-walled, in forebody. Pars prostatica long, tubular, surrounded by numerous gland cells, extends between distal end of seminal vesicle and sinus-sac. Metraterm protruding along with ejaculatory duct into sinus-sac forming hermaphroditic duct (Fig. 1E). Sinus-sac muscular. Sinus-organ muscular, conical, projecting into genital atrium. Genital pore ventro-median, posterior to pharynx, at level of intestinal bifurcation (observed only in five specimens).
Ovary transversely-oval, sinistral, post-testicular, at 1110 from posterior extremity. Oviduct, oötype, and Laurer’s canal not observed. Uterus well developed, coiled throughout much of hindbody and in forebody as far as level of sinus-sac. Vitellarium comprises two symmetrical, subglobular, multi-lobed, post-ovarian masses; right vitelline mass composed of 8–10 lobes; left vitelline mass composed of 7–9 lobes. Eggs numerous, small, tick-shelled, without opercular spines or filaments (Fig. 1D).
Excretory vesicle Y-shaped; bifurcation not observed; arms unite dorsally to oral sucker in forebody (Fig. 1B); excretory pore terminal.
Molecular Characterisation of the Digeneans
Four sequences (∼841 bp) for the nuclear 28S rRNA gene were obtained for D. ruber. The tree built using the newly generated sequences plus 20 sequences for species of Derogenes and the subfamily Halipeginae and Prosogonotrema bilabiatum Vigueras, 1940 as the outgroup yielded the topology shown in Fig. 2. There were a total of 688 positions in the final dataset. The general topology of the ML tree agreed with the taxonomic classification of the included species and distinct lineages. Species/lineages of Derogenes were recovered in five strongly supported reciprocally monophyletic clades: (i) D. ruber from C. lastoviza off Algeria; (ii) D. lacustris Tsuchida, Flores, Viozzi, Rauque et Urabe, 2021from Galaxias maculatus (Jenyns) off Argentina [31]; (iii) Lineage “D. varicus DV1” from fish hosts in the White and Barents seas [14]; (iv) Lineage “D. varicus DV2” from mollusc hosts in the White Sea [14]; and (v) Lineage “D. varicus DV3” from Eumicrotremus fedorovi Mandrytsa. in the Pacific Ocean [32]. All Derogenes spp. lineages (Derogeninae) clustered in a strongly supported clade distinct from that of the representatives of the Halipeginae.
Tree inferred using the maximum-likelihood method based on the 28S rDNA sequence data; only bootstrap values higher than 70 are indicated. The newly generated sequences are indicated in red. Lineages “Derogenes varicus DV1, DV2, DV3” and Derogenes lacustris are highlighted in differently colored boxes
The four newly generated 28S sequences of D. ruber were identical. They differed from Lineage “D. varicus DV1” from various fish hosts in the White and Barents seas (see above) by 2% (16 substitutions); from Lineage “D. varicus DV2” from Hippoglossoides platessoides (Fabricius) from North Sea and from a mollusc Buccinum scalariforme Møller. from the White Sea by 3% (20 substitutions); and from lineage “D. varicus DV3” by 2% (16 substitutions). Sequences of D. ruber differed from those of D. lacustris from G. maculatus (Jenyns) off Argentina by 9% (68 substitutions). Intraspecific/intralineage divergence for Derogenes spp./lineages ranged between 0 (for D. varicus lineages DV1, DV2, and DV3) and 1 substitution (for D. ruber and D. lacustris).
Five ITS2 sequences (∼566 bp) were obtained for D. ruber. The tree built using the newly generated sequences aligned with 12 sequences for Derogenes spp. and Prosogonotrema bilabiatum as the outgroup is shown in Fig. 3A. Derogenes ruber and the lineages “D. varicus DV1” from various fish hosts in the White and Barents seas and “D. varicus DV2” from the molluscs B. scalariforme, Amauropsis islandica (Gmelin) and Euspira pallida (Broderip & Sowerby) from the White and Barents seas clustered in reciprocally monophyletic groups with a maximum nodal support.
Trees inferred using the maximum-likelihood method based on the ITS2 rDNA and cox1 sequence data. A, ITS2 rDNA tree; only bootstrap values > 70 are indicated. The newly generated sequences are indicated in red. Lineages “D. varicus DV1 and “D. varicus DV2” are highlighted in differently colored boxes. There were no ITS2 sequences available for D. lacustris. B, cox1 tree; only bootstrap values > 70 are indicated. The newly generated sequences are indicated in red. Derogenes lacustris and lineages “D. varicus DV1”, “D. varicus DV2” are in different colors. There were no cox1 sequences available for the lineage “D. varicus DV3"
The five newly generated ITS2 sequences for D. ruber were also identical and differed from those for the lineage “D. varicus DV2” by 4% (16 substitutions) and from those for the lineage “D. varicus DV1” by 5% (21 substitutions). None of the taxa included in the analysis showed intraspecific/intralineage variation.
The two newly generated cox1 sequences of D. ruber (∼898 bp) were identical. We also included in the analysis four sequences of D. varicus (sensu stricto) from Merlangius merlangus (L.) from off Sweden [15]. The tree built using the newly generated sequences aligned with 22 sequences for Derogenes spp. and Didymocystis wedli Ariola, 1902 as the outgroup is shown in Fig. 3B. The species/lineages of Derogenes formed four reciprocally monophyletic groups with maximum support: (i) Derogenes lacustris from salmonids off Argentina; (ii) D. ruber from C. lastoviza off Algeria; (iii) Lineage “D. varicus DV1” from M. merlangus off Sweden, and fish hosts in the White and Barents seas [14]; and (iv) Lineage “D. varicus DV2” from mollusc hosts in the White Sea [32]. The intraspecific divergence between the newly generated cox1 sequences for D. ruber was 0.8% (7 substitutions). Sequences of D. ruber differed from the sequences for lineages “D. varicus DV1” and “D. varicus DV2” by 19% (158 substitutions) and 17% (135 substitutions), respectively. The largest genetic divergence was found between D. ruber and D. lacustris (23%; 186 substitutions). Intraspecific/intralineage divergence for Derogenes spp./lineages ranged between 0% (Lineage “D. varicus DV2”) and 2% (D. ruber: 0.8%; D. lacustris: 0.1–0.2; Lineage “D. varicus DV1”: 1%).
Discussion
Derogenes ruber was described from the gall bladder of the streaked gurnard C. lastoviza off Rovinj, Croatia, Adriatic Sea [16]. Although the original description of D. ruber was detailed, it lacked illustrations. The only subsequent illustration of this species is that of Sey [17], which barely shows any internal organs and omits any details of the terminal genitalia. Sey (1968) examined three specimens of a distinct host, T. lyra, and redescribed briefly D. ruber based on two specimens. Although the geographical distribution of the type-host, C. lastoviza, is wide, D. ruber has been reported only from the Central Mediterranean (Adriatic Sea off Croatia, type-locality in the original description [16] and later, from a different host [17] and recently from the type-host off Italy, based on A. Looss’s material) [15]. The latest record despite providing few morphometrical data and illustration did not include any genetic data. Derogenes ruber was reported from the type-host in the North-East Atlantic, off Azores, Canary and Cape Verde islands [18] and off Spain [19]. Consequently, this paper provides a detailed illustrated description of D. ruber and Algeria as a new locality for this digenean. Additionally, we genetically characterised for the first time D. ruber using the partial fragments of the nuclear 28S rRNA gene and ITS2, and the mitochondrial cox1 gene. Most sequences for Derogenes spp. available to date are those provided in an extensive study by Krupenko et al. [14] and Tsuchida et al. [31] who provided abundant data, corresponding to the “candidade” D. varicus species complex and D. lacustris, respectively. Krupenko et al. [14] have shown the existence of four groups (labelled as DV1-DV4) within the “candidade” D. varicus species complex; of these, they considered that two (DV1 and DV2) may belong to distinct species [14]. Recently, Bouguerche et al. [15] demonstrated that DV1 is in fact D. varicus sensu stricto.
Herein, the 28S rDNA analysis recovered D. ruber in a clade distinct from lineages “D. varicus DV1, DV2, and DV3” and the well-established species D. lacustris. The ITS2 analysis supported the monophyly of D. ruber, and lineages “D. varicus DV1” and “D. varicus DV2” and the cox1 tree yielded a similar topology. Although the sequences obtained herein were short affecting thus the alignment’s length, the analysis led to results similar to those of Krupenko et al. [14].
More importantly, the genetic distance for the cox1 gene between D. ruber and lineages “D. varicus DV1” and “D. varicus DV2” was 19% and 17%, respectively; D. ruber also differed from D. lacustris by 23%. These levels of genetic divergence agree well with previously reported interspecific divergence based on cox1 within the closely related halipegine derogenids ranging between 10.5–15.1% for Genarchopsis spp. [23] and 16.9–20.4% for Genarchopsis Ozaki, 1925 and Allogenarchopsis Urabe & Shimazu, 2013 [33]. Furthermore, the levels of interspecific genetic divergence are more than ten times greater than those for the intraspecific divergence for the mitochondrial “barcode” marker, thus supporting the recognition of D. ruber as a valid distinct species. The molecular data generated herein advance our knowledge on interspecific genetic variations within Derogenes and will help further efforts to untangle the D. varicus species complex and delimit the potentially cryptic species hidden under the single name “D. varicus”. Additionally, the morphometrical data of D. ruber from the type-host (Table 2) will help accessing interspecific morphological differences.
A problem arises when comparing D. ruber to a closely related species, D. latus Janiszewska, 1953, first described based on a single specimen in the intestine of Mullus barbatus Linnaeus from the same Adriatic locality as that of D. ruber, off Split, Croatia [34]. Derogenes latus was redescribed from the intestine of M. barbatus and Trisopterus capelanus (Lacépède) in the North Adriatic Sea [35] and from the gall bladder of M. surmuletus off Corsica (France), Western Mediterranean [10]. The redescription provided by Bartoli and Gibson [10] (based on accessible voucher material and serial sections) should undoubtfully be referred to as the most detailed modern redescription of D. latus. Derogenes latus has been frequently reported from its type-host in the Western Mediterranean, off Spain [36] and off France [37], and from a closely related host, M. surmuletus, in the Western Mediterranean (off France and Algeria) [37,38,39].
This species has also been reported on hosts other than Mullidae, mainly from S. scrofa (Scorpaenidae) in the Western Mediterranean, off Spain [40] and off France [41]; from L. mormyrus (Sparidae) off Montenegro, Adriatic Sea [42] and off Algeria, Western Mediterranean [43]. It was furthermore recorded from Sardinella aurita Valenciennes. (Dorosomatidae) off Algeria, Western Mediterranean [44] and from Phycis phycis (Linnaeus) (Phycidae) from the Western Mediterranean (off France) [41].
The taxonomic status of D. latus is uncertain. The distinction D. ruber and D. latus has been questioned [10], and the two species share a stout body, post-testicular vitellarium composed of two multi-lobed masses and a uterus occupying almost the entire body [10, 35, 42]. The type-hosts are, however, different: C. lastoviza for D. ruber [16] and M. barbatus for D. latus [34]. Overall, all morphometric data for D. ruber and D. latus overlapped (Tables 2, 3) except for specimens of D. latus from M. surmuletus and S. scrofa from the Western Mediterranean having larger eggs (see Table 3) and the two species clearly share the deeply loped shape of the vitelline masses. It is worth noting that a comparison of the present specimens of D. ruber with those of D. latus provided by Bartoli and Gibson [10] in the most detailed modern description based on accessible voucher material and serial sections and providing metrical data, revealed that, despite some overlaps, D. latus is generally larger than D. ruber (means 5581 × 2180 vs. 4348 × 1443 µm) with a longer forebody (mean 2523 vs. 1966 µm) and longer hindbody (mean 1926 vs. 1364 µm). Derogenes latus also differs from D. ruber in having a broadly longer pre-oral lobe (mean 126 vs. 39 µm), larger oral sucker (means 787 × 789 vs. 469 × 538 µm), larger ventral sucker (means 1136 × 1110 vs. 952 × 1022 µm), and larger pharynx (means 291 × 254 vs. 153 × 157 µm). Additionally, D. latus differs from D. ruber in having a longer pars prostatica (mean 790 vs. 565 µm), considerably larger testes (means 480 × 366 vs. 233 × 170 µm for right testis, 488 × 400 vs. 247 × 170 µm for left testis), larger ovary (means 511 × 341 vs. 293 × 183 µm), and larger vitelline masses (means 823 × 500 vs. 432 × 412 µm for right vitelline mass, 963 × 608 vs. 452 × 424 µm for left vitelline mass).
Bartoli and Gibson [10] convincingly highlighted the striking morphological similarity between D. latus and D. ruber and indicated that the egg size given by Sey [17] for D. ruber is probably an inaccuracy. They refrained from synonymising the two species formally until further studies of material from the type-hosts and localities are available. Although we found morphometric differences between the present material of D. ruber from the type-host and the material of D. latus described by Bartoli and Gibson [10], and given that the occurrence of a single Derogenes species in various hosts has been challenged by molecular data [14, 15, 31], and both D. lacustris and D. varicus sensu stricto (D. varicus lineage DV1 of Krupenko et al. [14]) had been genetically proven to occur in various hosts (see Fig. 3A), it is possible that D. ruber and D. latus are indeed synonymous, thus transforming D. ruber to a euryxenic species. However, since molecular data for D. latus are still lacking, we also refrained from synonymising the two species. The genetic data generated herein for D. ruber from its type-host will be certainly valuable for a future investigation of the synonymy of these two species.
Data availability
All relevant data are within the paper.
References
Gibson DI (2002) Family Derogenidae Nicoll, 1910. In: Gibson DI, Jones A, Bray RA (eds) Keys to the Trematoda, vol 1. CAB International and the Natural History Museum, Wallingford, pp 351–368
Dollfus R-P (1950) Hôtes et distribution géographique des cercaires cystophores [Hosts and geographic distribution of cystophorous cercariae]. Ann Parasitol Hum Comp 25(4):276–296. https://doi.org/10.1051/parasite/1950254276. (In French)
Nicoll W (1910) Studies on the structure and classification of the digenetic trematodes. J Cell Sci 2(211):391–488. https://doi.org/10.1242/jcs.s2-53.211.391
Skrjabin KI, Guschanskaja LH (1954) Suborder Hemiurata (Markevitsch) Skrjabin & Guschanskaja, 1954. First part. Osnovy Trematodologii 9:225–653 (In Russian)
Gibson D, Bray RA (1979) The Hemiuroidea: terminology, systematics and evolution. Bull Br Museum (Natural History) (Zoology Series) 36:35–146. https://doi.org/10.5962/BHL.PART.3604
Sokolov SG, Atopkin DM, Gordeev II, Shedko MB (2018) Phylogenetic position of the genus Gonocerca Manter, 1925 (Trematoda, Hemiuroidea), based on partial sequences of 28S rRNA gene and a reconsideration of taxonomic status of Gonocercinae Skrjabin et Guschanskaja, 1955. Parasitol Int 67(1):74–78. https://doi.org/10.1016/j.parint.2017.03.007
Manter HW (1940) Digenetic trematodes of fishes from the Galapagos Islands and the neighboring Pacific. Allan Hancock Pacific Expeditions 2:325–497
WoRMS. Derogenidae Nicoll, 1910. Accessed at: https://marinespecies.org/aphia.php?p=taxdetails&id=108468 on 2023–10–06. 2023.
Kostadinova A (1966) Gibson DI (2009) New records of rare derogenids (Digenea: Hemiuroidea) from Mediterranean sparids, including the description of a new species of Magnibursatus Naidenova, 1969 and redescription of Derogenes adriaticus Nikolaeva. Syst Parasitol 74(3):187–198. https://doi.org/10.1007/s11230-009-9214-6
Bartoli P, Gibson DI (1991) On Podocotyle scorpaenae, Poracanthium furcatum and Derogenes latus, three poorly known digenean parasites of western Mediterranean teleosts. Syst Parasitol 20(1):29–46. https://doi.org/10.1007/BF00009709
Pérez-del-Olmo A, Kostadinova A, Gibson DI (2016) The Mediterranean: high discovery rates for a well-studied trematode fauna. Syst Parasitol 93:249–256. https://doi.org/10.1007/s11230-016-9626-z
Bray RA, Diaz PE, Cribb TH (2016) Knowledge of marine fish trematodes of Atlantic and Eastern Pacific Oceans. Syst Parasitol 93:223–235. https://doi.org/10.1007/s11230-016-9629-9
Køie M (2000) Metazoan parasites of teleost fishes from Atlantic waters off the Faroe Islands. Ophelia 52(1):25–44. https://doi.org/10.1080/00785236.1999.10409417
Krupenko D, Kremnev G, Gonchar A, Uryadova A, Miroliubov A, Krapivin V, Skobkina O, Gubler A, Knyazeva O (2022) Species complexes and life cycles of digenetic trematodes from the family Derogenidae. Parasitology 149(12):1590–1606. https://doi.org/10.1017/s003118202200110x
Bouguerche C, Huston DC, Cribb TH, Karlsbakk E, Ahmed M, Holovachov O (2023) Hidden in the fog: morphological and molecular characterisation of Derogenes varicus sensu stricto (Trematoda, Derogenidae) from Sweden and Norway, and redescription of two poorly known Derogenes species. Parasite 30:35. https://doi.org/10.1051/parasite/2023030
Lühe M (1900) Über Distomen aus der Gallenblase von Mittelmeerfischen. Zool Anz 23(624):504–509
Sey O (1968) Parasitic helminths occurring in Adriatic fishes. Part 1. (Flukes). Acta Adriat 13:1–15
Kim M, Gijon-Botella H, Lopez-Roman R (1990) The Bunocotylidae, Derogenidae, Hemiuridae and Azygiidae of marine fishes. In: Doby JM (Ed) Proceedings of the Seventh International Congress of Parasitology, Paris August 20–24. In: Bulletin de la Société Francaise de Parasitologie.Vol. 8, Supplement 2. pp.733 (Abstract)
Cordero del Campillo M, Ordonez LC, Feo AR (1994) Indice-Catalogo de Zooparasitos Ibericos [Index-Catalogue of Iberian Zooparasites]. Dissertation, Universidad de Leon, Spain. 650 pp. (In Spanish)
Fischer W, Bauchot M-L and Schneider M (1987) Fiches FAO d’identification des espèces pour les besoins de la pêche. (Révision 1). Méditerranée et mer Noire. Zone de pêche 37. Volume II. Vertébrés. Publication préparée par la FAO, résultat d’un accord entre la FAO et la Commission des Communautés Européennes (Projet GCP/INT/422/EEC) financée conjointement par ces deux organisations. Rome, FAO, 2:761–1530
Kullander SO, Delling B (2012) Nationalnyckeln till Sveriges flora och fauna. Strålfeniga fiskar. Actinopterygii [The national key to Sweden's flora and fauna. Ray-finned fish. Actinopterygii], ed. L. Nyman and K. Jilg.: ArtDatabanken, SLU, Uppsala, 517 pp. (In Swedish)
Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol Phylogenet Evol 48(1):369–371. https://doi.org/10.1016/j.ympev.2008.03.024
Urabe M, Nishimura T, Shimazu T (2012) Taxonomic revision of three species of the genus Genarchopsis (Digenea: Hemiuroidea: Derogenidae) in Japan by molecular phylogenetic analyses. Parasitol Int 61(4):554–560. https://doi.org/10.1016/j.parint.2012.05.003
Pérez-Ponce de León G, Pinacho-Pinacho CD, Mendoza-Garfias B, Choudhury A, García-Varela M (2016) Phylogenetic analysis using the 28S rRNA gene reveals that the genus Paracreptotrema (Digenea: Allocreadiidae) is not monophyletic; description of two new genera and one new species. J Parasitol 102(1):131–142. https://doi.org/10.1645/15-815
García-Varela M, Nadler SA (2005) Phylogenetic relationships of Palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. J Parasitol 91(6):1401–1409. https://doi.org/10.1645/ge-523r.1
Morgan J, Blair D (1995) Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology 111(5):609–615. https://doi.org/10.1017/s003118200007709x
Cribb TH, Adlard RD, Bray RA (1998) A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). Int J Parasitol 28(11):1791–1795. https://doi.org/10.1016/s0020-7519(98)00127-1
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 30(22):3276–3278. https://doi.org/10.1093/bioinformatics/btu531
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120. https://doi.org/10.1007/bf01731581
Tsuchida K, Flores V, Viozzi G, Rauque C, Urabe M (2021) Hemiuroidean trematodes from freshwater Patagonian fishes: description of a new species, distribution and molecular phylogeny. Parasitol Res 120(4):1219–1232. https://doi.org/10.1007/s00436-020-06939-2
Gordeev II, Sokolov SG (2020) Helminths of Fedorov’s lumpsucker Eumicrotremus fedorovi Mandrytsa, 1991 (Actinopterygii: Cyclopteridae) in the Simushir Island area (Pacific Ocean). Parasitol Int 76:102075. https://doi.org/10.1016/j.parint.2020.102075
Urabe M, Shimazu T (2010) Allogenarchopsis gen. nov. (Digenea, Derogenidae, Halipeginae) parasitic in the intestine of freshwater fishes: a molecular and morphological study of adult and cercarial forms. Bulletin of the National Science Museum, Tokyo. Series A (Zoology) 39:119–130
Janiszewska J (1953) Some Adriatic Sea fish trematodes. Zoologica Poloniae 6:20–48
Paradižnik V, Radujković B (2007) Digenea trematodes in fish of the North Adriatic Sea. Acta Adriat 48(2):115–129
Ferrer-Maza D, Muñoz M, Lloret J, Faliex E, Vila S, Sasal P (2015) Health and reproduction of red mullet, Mullus barbatus, in the western Mediterranean Sea. Hydrobiologia 753:189–204. https://doi.org/10.1007/s10750-015-2205-5
Le Pommelet E, Bartoli P, Silan P (1997) Biodiversité des digènes et autres helminthes intestinaux des Rougets: synthèse pour Mullus surmuletus (Linné, 1758) et M. barbatus (L., 1758) dans le bassin méditerranéen [Biodiversity of digeneans and other intestinal helminths of Rougets: synthesis for Mullus surmuletus (Linnaeus, 1758) and M. barbatus (L., 1758) in the Mediterranean basin]. Annales Des Sciences Naturelles Zoologie 18:117–133 (In French)
Brahim Tazi NA, Meddour A, Bayssade-Dufour C, Zitouni B (2009) Investigation sur les parasites Digena de Mullus surmuletus Linné, 1758 dans le littoral Algérien [Investigation of digenean parasites of Mullus surmuletus Linné, 1758 in the Algerian coast]. Eur J Sci Res 25(3):448–462 (In French)
Hassani MM, Kerfouf A, Boutiba Z (2015) Checklist of helminth parasites of striped red mullet, Mullus surmuletus (Linnaeus, 1758)(Perciformes: Mullidae), caught in the Bay of Kristel, Algeria (western Mediterranean). Check list 11(1): 1504–1504. https://doi.org/10.15560/11.1.1504
Lozano C, Ubeda JM, De Rojas M, Ariza C, Guevara DC (2001) Estudio de digenidos de pèces marinos del sur de la Peninsula Iberica [Study of Digenea of marine fish from the south of the Iberian Peninsula]. Revista iberica de parasitologia 61(3–4):103–116
Ternengo S, Levron C, Mouillot D, Marchand B (2009) Site influence in parasite distribution from fishes of the Bonifacio Strait marine reserve (Corsica Island, Mediterranean Sea). Parasitol Res 104(6):1279–1287. https://doi.org/10.1007/s00436-008-1323-7
Radujković BM, Orecchia P, Paggi L (1989) Parasites des poissons marins du Monténégro: Digènes [Marine fish parasites of Montenegro: Digenes]. Acta Adriat 30(1/2):137–187 (In French)
Bellal A, Brahim Tazi NA, Charane M, Hadjou Z (2018) Gastrointestinal helminth parasites of Lithognathus mormyrus (Linnaeus,1758) (Perciformes Sparidae)in the Western Mediterranean Sea. Biodiversity J 9(1):9–18
Ramdani S, Trilles J-P, Ramdane Z (2020) Parasitic fauna of Sardinella aurita Valenciennes, 1847 from Algerian coast. Zool Ecol 30(1):101–108. https://doi.org/10.35513/21658005.2020.2.3
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DT (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33(7):733–755. https://doi.org/10.1016/s0020-7519(03)00049-3
Sokolov SG, Atopkin DM (1977) Gordeev II (2021) Phylogenetic position of the hemiuroid genus Paraccacladium Bray & Gibson, 1977 (Trematoda: Hemiuroidea) and the status of the subfamily Paraccacladiinae Bray & Gibson. Mar Biol Res 17(1):31–40. https://doi.org/10.1080/17451000.2021.1891252
Pérez-Ponce De León G, Hernández-Mena D (2019) Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’Tree of Life. J Helminthol 93(3):260–276. https://doi.org/10.1017/s0022149x19000191
Calhoun DM, Curran SS, Pulis EE, Provaznik JM, Franks JS (2013) Hirudinella ventricosa (Pallas, 1774) Baird, 1853 represents a species complex based on ribosomal DNA. Syst Parasitol 86(2):197–208. https://doi.org/10.1007/s11230-013-9439-2
Ahuir-Baraja AE, Fraija-Fernández N, Raga JA, Montero FE (2015) Molecular and morphological differentiation of two similar species of Accacoeliidae (Digenea): Accacladocoelium macrocotyle and A. nigroflavum from sunfish, Mola mola. J Parasitol 101(2):231–235. https://doi.org/10.1645/14-496.1
Acknowledgements
The authors thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions. The authors are grateful to all the participants of the International Bottom Trawl Survey cruises and the crew of the R/V “Svea” for their assistance with sampling in Sweden. The authors are grateful to all the staff of the Kristineberg Center for Marine Research and Innovation for their assistance. The authors also thank members of the Department of Zoology from the Swedish Museum of Natural History, Sweden especially Dr. Oleksandr Holovachov for kindly providing accession numbers. The authors are indebted to Dr. David Ian Gibson from the Natural History Museum, Life Sciences, United Kingdom for providing valuable literature and insightful comments. The authors thank Dr. Karin Tsuchida from The University of Shiga Prefecture, Japan and Verónica Flores from Laboratorio de Parasitología, INIBIOMA (CONICET–Univ. Nac. del Comahue), Argentina and Dr. Darya Krupenko from Saint Petersburg University, Russia for kindly helping with the literature. The authors would like to thank the two anonymous reviewers who provided several thoughtful suggestions.
Funding
Open access funding provided by Swedish Museum of Natural History. Chahinez Bouguerche was supported individually by a framework agreement projects: 1. the DeepBlue Project: Distance Crossborder Traineeship Programme co-financed by “The European Maritime and Fisheries Fund (EMFF)” for the analysis, interpretation of data, and the writing of the manuscript, 2. “The Ocean Fellowship” (edition 2021), offered by TBA21–Academy and held at Ocean Space in Venice and 3. the Swedish Taxonomy Initiative, Artdatabanken, Swedish University of Agricultural Sciences within the scope of the project “Taxonomy and systematics of digenetic trematodes parasitising fishes of Sweden” (dha 2019.4.3–48). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
KG collected specimens, measured and drew some specimens, and approved the final draft. CB obtained funding, conceived and designed the experiments, drew specimens, prepared figures and tables, authored or reviewed drafts of the paper, and approved the final draft. GP-PL conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft. MA obtained sequences, authored drafts of the paper, and approved the final draft. FT approved the final draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethical Approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gharbi, K., Bouguerche, C., Ahmed, M. et al. Redescription and Molecular Characterisation of Derogenes ruber Lühe, 1900 (Hemiuroidea: Derogenidae) from Chelidonichthys lastoviza (Scorpaeniformes: Triglidae) in the Western Mediterranean. Acta Parasit. 69, 309–323 (2024). https://doi.org/10.1007/s11686-023-00749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00749-z