Abstract
Generalized fractional anisotropy (GFA) can eliminate the crossing fiber effect, which may be more reflective of brain tissue changes in patients with cerebral small vessel disease (CSVD). This study aimed to explore the alterations of structural networks based on GFA and its relationship with cognitive performance in CSVD patients. We recruited 50 CSVD patients which were divided into two groups: cognitive impairment (CSVD-CI) and normal cognition (CSVD-NC), and 22 healthy controls (HCs). All participants underwent the Montreal Cognitive Assessment (MoCA) and MRI examinations. The structural topological properties were compared among the three groups. The correlation between these structural alterations and MoCA was analyzed. Compared with HCs, significantly decreased nodal efficiency and connectivity were detected in the corticothalamic pathways in both patient groups, of which some were significantly decreased in CSVD-CIs compared with CSVD-NCs. Moreover, both patient groups exhibited global network disruption including decreased global efficiency and increased characteristic path length compared with HCs. Furthermore, the nodal efficiency in the right pallidum positively correlated with MoCA in CSVD-NCs controlling for nuisance variables (r = 0.471, p = 0.031). The alterations in corticothalamic pathways indicated that the brain structural network underwent extensive disruption, providing evidence for the consideration of CSVD as a global brain disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral small vessel disease (CSVD) is the major cause of vascular cognitive decline and dementia (Jellinger, 2007; Pantoni, 2010). It refers to a series of clinical, imaging, and pathological syndromes caused by various etiologies affecting small blood vessels in the brain (Ter Telgte et al., 2018). Currently, the diagnosis of CSVD relies mainly on conventional imaging. However, the subtle changes in brain structure may occur early before the initial onset of conventional neuroimaging features.
Ed Bullmore et al. highlighted the growing relevance of graph theory analysis approaches in understanding the physics of brain connectome (Bullmore & Sporns, 2009). In particular, structural connectivity exhibits greater sensitivity than functional connectivity when assessing nodal and global network parameters (Zhu et al., 2022), and it is also considered as a predictor of functional connectivity (Bullmore & Sporns, 2009).
Advances in diffusion tensor imaging (DTI) have enabled the examination of microstructural changes in white matter, preceding the occurrence of visible lesions on conventional magnetic resonance imaging (MRI) in patients with CSVD. Specifically, fractional anisotropy (FA) is the most widely used diffusion parameter. However, FA is limited to representing a single fiber direction (Alexander et al., 2001), thus leading to an underestimation of the influence of crossing fibers (Winston, 2012). The emerging diffusion parameter generalized fractional anisotropy (GFA), derived from the q-ball imaging linear reconstruction scheme, addresses the problem of fiber crossing in deep white matter pathways and subcortical margins in vivo (Tuch, 2004). It is considered to be robust and stable compared with FA (Ji et al., 2019; Ueda et al., 2016).
We aimed to explore the structural topological alterations in patients with CSVD using GFA and to examine the relationship between these alterations and cognitive performance in CSVD patients. In the present study, it was hypothesized that the corticothalamic pathways might exhibit structural alterations in patients with CSVD.
Materials and methods
Participants
We enrolled 50 asymptomatic CSVD patients that met the following criteria: (a) age ≥ 50 years, and (b) one or more CSVD neuroimaging biomarkers on MRI with CSVD score ≥ 1. Neuroimaging biomarkers included white matter hyperintensity (WMH) of presumed vascular, recent small subcortical infarcts, lacune, perivascular space (PVS), cerebral microbleed (CMB) and brain atrophy (Duering et al., 2023; Wardlaw et al., 2013). The CSVD score was an assessment of the total MRI burden for CSVD neuroimaging biomarkers, allocating 1 point each for the presence of: (a) deep WMH (Fazekas score ≥ 2) or periventricular WMH (Fazekas score = 3), (b) one or more lacunes, (c) one or more CMBs, and (d) moderate to severe PVS in the basal ganglia region (grade 2–4) (Smith et al., 2012; Staals et al., 2014). In addition, 22 age- and sex-matched healthy controls (HCs) were included from the community.
The exclusion criteria were as follows: (a) history of genetic CSVD and cerebral amyloid angiopathy, (b) participants with transient ischemic attack within 3 months, (c) history of intracranial atherosclerotic disease, (d) history of psychiatric disorders, (e) other neurological diseases, such as multiple sclerosis and Parkinson disease, (f) intracranial hemorrhage or occupying lesions, (g) visual or auditory impairment, and (h) claustrophobia or other contraindications to MRI.
All participants underwent MRI examinations and the Montreal Cognitive Assessment (MoCA). The MoCA score was corrected for education level, increasing by 1 point when the education level of the participant was ≤ 12 years, with a maximum score of 30. The patients were divided into two groups: 29 CSVD patients with cognitive impairment (CSVD-CI) and 21 CSVD patients with normal cognition (CSVD-NC), using a cutoff MoCA score of 26 (Nasreddine et al., 2005).
This study was approved by the Ethics Committee Boards of Beijing Chaoyang Hospital, Capital Medical University and written informed consent was obtained from each participant.
Image acquisition
The participants underwent brain MRI on a 3.0 T scanner (MAGNETOM Skyra, Siemens Healthcare) with a 20-channel head coil. The T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) imaging parameters were: repetition time (TR) = 2300 ms, echo time (TE) = 2.32 ms, inversion time (TI) = 450 ms, slice thickness = 1 mm, gap = 50%, flip angle = 8°, field of view (FOV) = 256 × 256 mm2, number of slices = 192. The T2 imaging parameters were: TR = 3900 ms, TE = 99 ms, slice thickness = 5 mm, number of slices = 20. T2-weighted fluid attenuated inversion recovery (FLAIR) imaging parameters were: TR = 8000 ms, TE = 81 ms, slice thickness = 5 mm, number of slices = 20. DTI was acquired in the anterior-to-posterior phase-encoding direction, and b-value for non-zero gradient volumes was 1000 s/ mm2 along 64 gradient directions: acquisition matrix = 74 × 74, axial slices = 76, TR = 5200 ms, TE = 74 ms. Susceptibility weighted imaging (SWI) parameters were: TR = 28 ms, TE = 20 ms, slice thickness = 2 mm, FOV = 220 × 220 mm2.
Data preprocessing
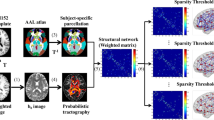
Data preprocessing was performed using DSI Studio (http://dsi-studio.labsolver.org). First, the DTI data were corrected for motion and eddy current distortion. Then the images were reconstructed in the Montreal Neurological Institute (MNI) space using q-space diffeomorphic reconstruction, which were rotated and scaled to the space of T1MPRAGE with b-table also rotated accordingly. A deterministic fiber tracking algorithm was used with augmented tracking strategies to improve reproducibility (Yeh, 2020). A seeding region was placed at whole brain, where a total of 1,000,000 seeds were placed. The anisotropy threshold was randomly selected. The angular threshold was randomly selected from 15 degrees to 90 degrees and the step size was randomly selected from 0.5 voxel to 1.5 voxels. Tracks with length shorter than 10 or longer than 200 mm were discarded.
Network node and edge definition
Automated anatomical labeling (AAL) atlas was used as the brain parcellation (Rolls et al., 2020), and the connectivity matrix was calculated using GFA and FA of the connecting tracks. For the network node definition, the whole brain was divided into 166 cortical or subcortical regions by the AAL3 atlas with each region as a node. We defined the average GFA and FA value of the white matter fibers between two nodes as the weight of the edge (Zhou et al., 2022).
Network analysis
All network parameters, including nodal and global topological network feature parameters, were obtained using the graph theoretical network analysis (GRETNA) toolbox (https://www.nitrc.org/projects/gretna). In this study, weighted networks were chosen to identify subtle network organization (Martensson et al., 2018). Brain networks are often compared with random networks to test whether they are configured with a significant non-random topology. Random networks are generated by the Markov wiring algorithm (Maslov & Sneppen, 2002), which preserves the same number of nodes and edges and the same degree distribution as the real brain network. The sparse thresholds used in this study ranged from 0.1 to 0.3 with an interval of 0.01.
At the nodal level, we calculated nodal efficiency, the inverse of the shortest path length of a subnetwork in which the node participated. This quantified the importance of nodes for information communication within the network (Fox & King, 2018). In global level, we calculated the area under the curve (AUC) values of global network parameters and their values at various thresholds: (1) global efficiency (Eglob), measured the efficiency of parallel message transmission in the whole-brain network; (2) the characteristic path length (Lp), calculating the average shortest path length between all pairs of nodes in the network; (3) gamma (γ), normalized clustering coefficient; (4) lambda (λ), normalized Lp; (5) sigma (σ), small-worldness, γ/λ (Reijmer et al., 2013; Rubinov & Sporns, 2010; Wang et al., 2011).
Statistical analysis
Demographics and clinical characteristics were analyzed using SPSS software (version 26.0, IBM). We assessed whether demographic data and global network parameters were statistically disparate among three groups. One-way analysis of variance (ANOVA) was chosen if continuous data followed a normal distribution and homogeneous variance, and Bonferroni post hoc tests were performed. In the case of non-normal or heterogeneous variance, Kruskal-Wallis tests were used. For categorical variables, chi-square tests were performed. We used a statistical significance level of p < 0.05.
One-way ANOVA was performed for nodal efficiency among the three groups to obtain the significantly different brain regions, which were defined as regions of interest (ROIs). We performed the false discovery rate (FDR) correction on the ROIs, using the built-in function in MATLAB: mafdr (p_vector,‘BHFDR’, true). Further least significant difference post-hoc tests were used for pairwise comparisons. In the CSVD-CI and CSVD-NC group, partial correlation and regression analyses were performed to test the relationship between nodal efficiency and MoCA for brain regions, considering age, sex, and education as covariates. The above analyses were performed in SPSS 26.0. The connectivity difference was calculated using the two-sample t-test followed by network-based statistics (NBS) correction with 5,000 permutations for each participant using the GRETNA toolbox. The significance level was set at p < 0.05 corrected.
Results
Demographic and clinical characteristics of participants
The demographic characteristics and clinical parameters of each group are summarized in Table 1. The CSVD-CI group had significantly decreased MoCA than the CSVD-NC and HC groups (p < 0.001). No significant differences in age, sex, and neuroimaging features were observed among the three groups.
Node-based analysis
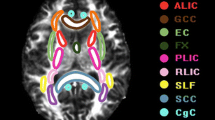
Nineteen brain regions had significantly different nodal efficiency among the three groups (p < 0.05). Compared with the HC group, the CSVD-NC group demonstrated decreased nodal efficiency in the bilateral dorsolateral superior frontal gyrus (SFG) (L, p = 0.004; R, p = 0.037), left hippocampus (p = 0.003), left fusiform gyrus (p = 0.014), bilateral putamen (L, p = 0.013; R, p = 0.035) and right pulvinar medial thalamus (tPuM) (p = 0.020). Besides, the CSVD-CI group further showed considerably decreased nodal efficiency in the bilateral parahippocampal gyrus (PHG) (L, p = 0.007; R, p = 0.014), right paracentral lobule (p = 0.009), right pallidum (p = 0.008), right ventral anterior thalamus (tVA) (p = 0.008), left lateral geniculate thalamus (tLGN) (p = 0.013), right medial geniculate thalamus (tMGN) (p = 0.014), bilateral pulvinar anterior thalamus (tPuA) (L, p = 0.002; R, p = 0.003), right pulvinar inferior thalamus (tPuI) (p = 0.008) and left pregenual anterior cingulate cortex (ACCpre) (p = 0.001). Moreover, compared with the CSVD-NC group, the CSVD-CI group exhibited significantly decreased nodal efficiency in the right PHG (p = 0.010), left calcarine (p = 0.007), right paracentral lobule (p = 0.004), right tMGN (p = 0.012), right tPuA (p = 0.035), and left ACCpre (p = 0.043) (Table 2; Fig. 1).
Structural connectome with significant differences between two groups, (A) CSVD-CI with HC, (B) CSVD-NC with HC, and (C) CSVD-CI with CSVD-NC. The first row demonstrates altered regions and connections of structural networks. Nodes represent brain areas with remarkably different values of nodal efficiency, and edges represent connections between differential brain regions. The second row demonstrates significantly differential regions of nodal efficiency. *, p < 0.05 for comparison between groups; **, p < 0.01. In the third row, the heat maps demonstrate structural connections between the two brain regions and their strength. ACCpre, pregenual of anterior cingulate cortex; CAL, calcarine fissure and surrounding cortex; FFG, fusiform gyrus; HIP, hippocampus; PAL, pallidum; PCL, paracentral lobule; PHG, parahippocampal gyrus; PUT, putamen; SFG, superior frontal gyrus; tLGN, lateral geniculate thalamus; tMGN, medial geniculate thalamus; tPuA, pulvinar anterior thalamus; tPuI, pulvinar inferior thalamus; tPuM, pulvinar medial thalamus; tVA, ventral anterior thalamus; L, left; R, right; CSVD-CI, CSVD patients with cognitive impairment; CSVD-NC, CSVD patients with normal cognition; HC, healthy control
The connectivity alterations could be observed in the heat map (Fig. 1). Most of the structural connections were decreased in both patient groups compared with the HC group. Nevertheless, the network connections significantly increased between right PHG and right tVA and tPuI in the CSVD-CI group compared with the HC group and between the right PHG and right tPuA in the CSVD-CI group compared with the CSVD-NC group.
We performed brain network analysis based on FA using the same pipeline and compared with GFA. There was a good consistency between the results of FA and GFA, with 16 overlapping brain regions (Fig. 2). However, there were some regions existing only in FA, including the left precentral gyrus, left middle frontal gyrus, right supplementary motor area, left medial superior frontal gyrus, right superior frontal gyrus, medial orbital, right precuneus, bilateral caudate nucleus, left pallidum, left lateral posterior thalamus, left tVA, left ventral lateral thalamus, right tLGN, left tMGN, left tPuM, and right pulvinar lateral thalamus. The additional regions using GFA included the bilateral PHG, left fusiform gyrus, and right tPuI.
Structural connectome with significant differences between two groups respectively based on GFA and FA. ACCpre, pregenual of anterior cingulate cortex; CAU, caudate nucleus; FFG, fusiform gyrus; HIP, hippocampus; MFG, middle frontal gyrus; ORBsupmed, superior frontal gyrus, medial orbital; PAL, pallidum; PCL, paracentral lobule; PCUN, precuneus; PHG, parahippocampal gyrus; PreCG, precentral gyrus; PUT, putamen; SFG, superior frontal gyrus; SFGmed, superior frontal gyrus, medial; SMA, supplementary motor area; tLGN, lateral geniculate thalamus; tLP, lateral posterior thalamus; tMGN, medial geniculate thalamus; tPuA, pulvinar anterior thalamus; tPuI, pulvinar inferior thalamus; tPuL, pulvinar lateral thalamus; tPuM, pulvinar medial thalamus; tVA, ventral anterior thalamus; tVL, ventral lateral thalamus; L, left; R, right; CSVD-CI, CSVD patients with cognitive impairment; CSVD-NC, CSVD patients with normal cognition; HC, healthy control
Global network analysis
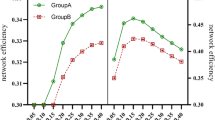
Increased aLp (p = 0.024) and decreased aEglob (p = 0.020) based on GFA were observed in the CSVD-CI and CSVD-NC group compared with the HC group (Table 3). All three groups of brain structural networks conformed to small-world properties with γ > 1.1, λ ≈ 1, and σ > 1.
Correlation analysis
The nodal efficiency in the right pallidum was positively associated with MoCA in the CSVD-NC group (r = 0.471, p = 0.031) (Fig. 3). No significant correlations were observed between global network parameters and MoCA.
The correlation between nodal efficiency of the right pallidum and MoCA in CSVD-NC and CSVD-CI groups. Of note, the coordinates of both the X axis (MoCA) and Y axis (nodal efficiency of the right pallidum) do not reflect the initial values of these variables when taking age, sex and education as covariates. MoCA, the Montreal Cognitive Assessment
Discussion
The present study explored the subtle changes in structural connectivity in patients with CSVD. First, the brain regions with decreased nodal efficiency and disrupted structural connectivity were predominantly concentrated in corticothalamic pathways in both patient groups. Second, structural connectivity increased between the right PHG and thalamus subregions in the CSVD-CI group, compared with the CSVD-NC and HC groups. Finally, the global topological organization in CSVD patients was disrupted, as indicated by decreased global efficiency and increased characteristic path length.
Damage to regions in CSVD patients may be associated with cognitive decline and can make these regions more susceptible to disease. Based on GFA and FA, brain regions exhibiting significantly decreased nodal efficiency were primarily concentrated in the thalamus, frontal lobes, hippocampus, and basal ganglia in both patient groups compared with HCs. Notably, the efficiency of information processing in brain regions within the corticothalamic pathways reduced in vascular cognitive decline (Jellinger, 2007). These pathways consisted of interconnected networks responsible for processing decision-making, cognition, associative, and sensorimotor information (Fischer, 2021; Vich et al., 2022). In addition, amyloid deposition is believed to contribute to the impairment of information transmission and to have a synergistic effect with structural brain disruption. Together, these factors can lead to extensive breakdown of brain tissue, eventually leading to cognitive impairment (Son et al., 2022). There were more regions decreased in FA, which might indicate the notion that FA values were underestimated in the regions containing crossing fibers (Winston, 2012). Moreover, the locations of these brain regions were also consistent with previous studies, focusing on the centrum semiovale, superior longitudinal fasciculus, and thalamus (Oouchi et al., 2007; Szeszko et al., 2018). The results based on GFA could be more sensitive than FA due to observation of the PHG and FFG, which suggested us to focus on the role of these brain regions in CSVD patients.
Although a few CSVD patients maintain normal cognition, they might still experience structural and functional alterations. We speculated that decreased nodal efficiency in corticothalamic pathways could serve as an early indicator of CSVD-related cognitive decline. The nodal efficiency of the right pallidum was positively associated with MoCA in CSVD-NC patients. The pallidum plays a pivotal role as a “transfer station” connecting the cortex to the thalamus to participate in cortical regulation, including motor control, associative learning, and planning (Obeso et al., 2008). Our findings supported the role of the pallidum in cognition of CSVD patients. Furthermore, the CSVD-CI group exhibited more regions with substantial decreases in nodal efficiency compared with the CSVD-NC and HC groups. These regions, including the left PHG, right pallidum, right tVA, left tLGN, left tPuA, and right tPuI, were part of the subcortical network. This network is pivotal in facilitating large-scale neural communication, with the basal ganglia and thalamus playing crucial roles as part of a core circuit supporting the integration of neural information (Bell & Shine, 2016). In the current study, the disrupted corticothalamic structural connectivity in patients with CSVD might suggest the potential pathophysiological mechanisms in this disease.
This study demonstrated that corticothalamic dysconnectivity was associated with global cognition (Chen et al., 2019). Of note, there were increased connections between the right PHG and right striato-thalamic regions in the CSVD-CI group, which may be a compensation or reorganization between networks (Filippi & Rocca, 2003). The PHG, a part of memory system, is involved in complex cognitive performance (Kesslak et al., 1991). The striato-thalamic pathway is critical for information transmission from cortex and subcortex. Ter Telgte et al. suggested that the efficiency of structural networks was a form of brain reserve preventing clinical deterioration. The present study demonstrated that the brain could use alternative connectivity or connection enhancement to compensate for the disruption of white matter microstructure.
This study observed that both CSVD patients and the HCs conformed to the small-worldness, which was consistent with the previous studies based on FA or fiber number (Wen et al., 2017; Xin et al., 2022). The small-world network aims to maximize efficiency and minimize cost (Bassett & Bullmore, 2006). However, small-worldness does not fully reflect and generalize the complex brain network (Bassett & Bullmore, 2017). Consistent with a longitudinal demonstrating study that network efficiency at baseline predicted mortality and cognitive decline (Tuladhar et al., 2020), a decrease in global efficiency and an increase in characteristic path length indicated connectivity alterations in both patient groups compared with HCs. This might imply a decline in information transfer efficiency and functional integration ability among brain regions in this disease.
This study had several limitations. First, MoCA reflects only overall cognition. Comprehensive neuropsychological tests will be required in the future to explore deficits specific to sub-domains. Second, the sample size was relatively small. Future studies with larger sample sizes are needed to validate the current findings. Third, the analyses were cross-sectional, which made it difficult to detect temporal and causal relationships among the variables. Hence, longitudinal studies are necessary to explore the pattern of disease evolution.
Conclusions
This study demonstrated that efficient communication in corticothalamic pathways was disturbed in CSVD patients, of which the structural connectivity was strongly affected in CSVD-CIs. The extensive disruption of structural network provided evidence for the consideration of CSVD as a global brain disease. We also found the compensation phenomenon in CSVD-CIs and provided evidence for a role of the right pallidum in cognition in CSVD-NCs.
Data availability
Not applicable.
References
Alexander, A. L., Hasan, K. M., Lazar, M., Tsuruda, J. S., & Parker, D. L. (2001). Analysis of partial volume effects in diffusion-tensor MRI. Magnetic Resonance in Medicine, 45(5), 770–780. https://doi.org/10.1002/mrm.1105.
Bassett, D. S., & Bullmore, E. (2006). Small-world brain networks. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 12(6), 512–523. https://doi.org/10.1177/1073858406293182.
Bassett, D. S., & Bullmore, E. T. (2017). Small-world brain networks revisited. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 23(5), 499–516. https://doi.org/10.1177/1073858416667720.
Bell, P. T., & Shine, J. M. (2016). Subcortical contributions to large-scale network communication. Neuroscience and Biobehavioral Reviews, 71, 313–322. https://doi.org/10.1016/j.neubiorev.2016.08.036.
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186–198. https://doi.org/10.1038/nrn2575.
Chen, X., Wang, J., Shan, Y., Cai, W., Liu, S., Hu, M., & Lu, Z. (2019). Cerebral small vessel disease: Neuroimaging markers and clinical implication. Journal of Neurology, 266(10), 2347–2362. https://doi.org/10.1007/s00415-018-9077-3.
Duering, M., Biessels, G. J., Brodtmann, A., Chen, C., Cordonnier, C., de Leeuw, F. E., & Wardlaw, J. M. (2023). Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurology. https://doi.org/10.1016/S1474-4422(23)00131-X.
Filippi, M., & Rocca, M. A. (2003). Disturbed function and plasticity in multiple sclerosis as gleaned from functional magnetic resonance imaging. Current Opinion in Neurology, 16(3), 275–282. https://doi.org/10.1097/01.wco.0000073927.19076.60.
Fischer, P. (2021). Mechanisms of Network interactions for flexible cortico-basal ganglia-mediated Action Control. eNeuro, 8(3), ENEURO0009–0021. https://doi.org/10.1523/ENEURO.0009-21.2021.
Fox, M. E., & King, T. Z. (2018). Functional connectivity in adult brain tumor patients: A systematic review. Brain Connectivity, 8(7), 381–397. https://doi.org/10.1089/brain.2018.0623.
Jellinger, K. A. (2007). The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathologica, 113(4), 349–388. https://doi.org/10.1007/s00401-006-0185-2.
Ji, E., Guevara, P., Guevara, M., Grigis, A., Labra, N., Sarrazin, S., & Houenou, J. (2019). Increased and decreased superficial White Matter Structural Connectivity in Schizophrenia and Bipolar Disorder. Schizophrenia Bulletin, 45(6), 1367–1378. https://doi.org/10.1093/schbul/sbz015.
Kesslak, J. P., Nalcioglu, O., & Cotman, C. W. (1991). Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer’s disease. Neurology, 41(1), 51–54. https://doi.org/10.1212/wnl.41.1.51.
Martensson, G., Pereira, J. B., Mecocci, P., Vellas, B., Tsolaki, M., Kloszewska, I., & Westman, E. (2018). Stability of graph theoretical measures in structural brain networks in Alzheimer’s disease. Scientific Reports, 8(1), 11592. https://doi.org/10.1038/s41598-018-29927-0.
Maslov, S., & Sneppen, K. (2002). Specificity and stability in topology of protein networks. Science, 296(5569), 910–913. https://doi.org/10.1126/science.1065103.
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
Obeso, J. A., Rodríguez-Oroz, M. C., Benitez-Temino, B., Blesa, F. J., Guridi, J., Marin, C., & Rodriguez, M. (2008). Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Movement Disorders, 23(Suppl 3), 548–559. https://doi.org/10.1002/mds.22062.
Oouchi, H., Yamada, K., Sakai, K., Kizu, O., Kubota, T., Ito, H., & Nishimura, T. (2007). Diffusion anisotropy measurement of brain white matter is affected by voxel size: Underestimation occurs in areas with crossing fibers. Ajnr. American Journal of Neuroradiology, 28(6), 1102–1106. https://doi.org/10.3174/ajnr.A0488.
Pantoni, L. (2010). Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurology, 9(7), 689–701. https://doi.org/10.1016/S1474-4422(10)70104-6.
Reijmer, Y. D., Leemans, A., Caeyenberghs, K., Heringa, S. M., Koek, H. L., & Biessels, G. J. (2013). Disruption of cerebral networks and cognitive impairment in Alzheimer disease. Neurology, 80(15), 1370–1377. https://doi.org/10.1212/WNL.0b013e31828c2ee5. & Utrecht Vascular Cognitive Impairment Study
Rolls, E. T., Huang, C. C., Lin, C. P., Feng, J., & Joliot, M. (2020). Automated anatomical labelling atlas 3. Neuroimage, 206, 116189. https://doi.org/10.1016/j.neuroimage.2019.116189.
Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage, 52(3), 1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003.
Smith, E. E., Schneider, J. A., Wardlaw, J. M., & Greenberg, S. M. (2012). Cerebral microinfarcts: The invisible lesions. Lancet Neurology, 11(3), 272–282. https://doi.org/10.1016/S1474-4422(11)70307-6.
Son, S. J., Hong, C. H., Kim, N. R., Choi, J. W., Roh, H. W., Lee, H., & Park, B. (2022). Structural covariance changes in major cortico-basal ganglia and thalamic networks in amyloid-positive patients with white matter hyperintensities. Neurobiology of Aging, 117, 117–127. https://doi.org/10.1016/j.neurobiolaging.2022.05.010.
Staals, J., Makin, S. D., Doubal, F. N., Dennis, M. S., & Wardlaw, J. M. (2014). Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology, 83(14), 1228–1234. https://doi.org/10.1212/WNL.0000000000000837.
Szeszko, P. R., Tan, E. T., Ulug, A. M., Kingsley, P. B., Gallego, J. A., Rhindress, K., & Marinelli, L. (2018). Investigation of superior longitudinal fasciculus fiber complexity in recent onset psychosis. Progress in Neuropsychopharmacology and Biological Psychiatry, 81, 114–121. https://doi.org/10.1016/j.pnpbp.2017.10.019.
Ter Telgte, A., van Leijsen, E. M. C., Wiegertjes, K., Klijn, C. J. M., Tuladhar, A. M., & de Leeuw, F. E. (2018). Cerebral small vessel disease: From a focal to a global perspective. Nat Rev Neurol, 14(7), 387–398. https://doi.org/10.1038/s41582-018-0014-y.
Tuch, D. S. (2004). Q-ball imaging. Magnetic Resonance in Medicine, 52(6), 1358–1372. https://doi.org/10.1002/mrm.20279.
Tuladhar, A. M., Tay, J., van Leijsen, E., Lawrence, A. J., van Uden, I. W. M., Bergkamp, M., & De Leeuw, F. E. (2020). Structural network changes in cerebral small vessel disease. Journal of Neurology, Neurosurgery and Psychiatry, 91(2), 196–203. https://doi.org/10.1136/jnnp-2019-321767.
Ueda, R., Yamada, N., Kakuda, W., Abo, M., & Senoo, A. (2016). White matter structure and clinical characteristics of stroke patients: A diffusion tensor MRI study. Brain Research, 1635, 61–70. https://doi.org/10.1016/j.brainres.2015.12.059.
Vich, C., Clapp, M., Rubin, J. E., & Verstynen, T. (2022). Identifying control ensembles for information processing within the cortico-basal ganglia-thalamic circuit. Plos Computational Biology, 18(6), e1010255. https://doi.org/10.1371/journal.pcbi.1010255.
Wang, J. H., Zuo, X. N., Gohel, S., Milham, M. P., Biswal, B. B., & He, Y. (2011). Graph theoretical analysis of functional brain networks: Test-retest evaluation on short- and long-term resting-state functional MRI data. PLoS One, 6(7), e21976. https://doi.org/10.1371/journal.pone.0021976.
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., & nEuroimaging, S. T. f. R. V. c. o. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology, 12(8), 822–838. https://doi.org/10.1016/S1474-4422(13)70124-8.
Wen, H., Liu, Y., Rekik, I., Wang, S., Zhang, J., Zhang, Y., & He, H. (2017). Disrupted topological organization of structural networks revealed by probabilistic diffusion tractography in Tourette syndrome children. Human Brain Mapping, 38(8), 3988–4008. https://doi.org/10.1002/hbm.23643.
Winston, G. P. (2012). The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg, 2(4), 254–265. https://doi.org/10.3978/j.issn.2223-4292.2012.12.05.
Xin, H., Wen, H., Feng, M., Gao, Y., Sui, C., Zhang, N., & Guo, L. (2022). Disrupted topological organization of resting-state functional brain networks in cerebral small vessel disease. Human Brain Mapping, 43(8), 2607–2620. https://doi.org/10.1002/hbm.25808.
Yeh, F. C. (2020). Shape analysis of the human association pathways. Neuroimage, 223, 117329. https://doi.org/10.1016/j.neuroimage.2020.117329.
Zhou, J., Jiang, X., Zhou, Y., Zhu, Y., Jia, L., Sun, T., & Tang, Y. (2022). Distinguishing major depressive disorder from bipolar disorder in remission: A brain structural network analysis. Journal of Affective Disorders, 319, 8–14. https://doi.org/10.1016/j.jad.2022.08.102.
Zhu, H., Zuo, L., Zhu, W., Jing, J., Zhang, Z., Ding, L., & Li, Z. (2022). The distinct disrupted plasticity in structural and functional network in mild stroke with basal ganglia region infarcts. Brain Imaging Behav, 16(5), 2199–2219. https://doi.org/10.1007/s11682-022-00689-8.
Funding
This study was funded by the National Natural Science Foundation of China (grant number: 62076169), Interdisciplinary Clinical Research Innovation Team Project of Beijing Chaoyang Hospital (grant number: CYDXK202207), Beijing Hospitals Authority’s Ascent Plan (grant number: DFL20220303) and Beijing Key Specialists in Major Epidemic Prevention and Control.
Author information
Authors and Affiliations
Contributions
Xiuqin Jia and Qi Yang, Conceptualization and designation of study. Xuejia Jia and Yingying Li, Material preparation, data collection and analysis. Xuejia Jia drafted the manuscript. All the authors reviewed it and provided feedback.
Corresponding authors
Ethics declarations
This study was approved by the Beijing Chaoyang Hospital Ethics Review Board. It met the guidelines of Capital Medical University, which abides by the Helsinki Declaration on ethical principles for medical research involving human participants. Informed consent was obtained from all participants involved in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, X., Li, Y., Jia, X. et al. Structural network disruption of corticothalamic pathways in cerebral small vessel disease. Brain Imaging and Behavior (2024). https://doi.org/10.1007/s11682-024-00889-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11682-024-00889-4