Abstract
Structural and functional changes in cortical and subcortical regions have been reported in behavioral variant frontotemporal dementia (bvFTD), however, a multimodal approach may provide deeper insights into the neural correlates of neuropsychiatric symptoms. In this multicenter study, we measured cortical thickness (CTh) and subcortical volumes to identify structural abnormalities in 37 bvFTD patients, and 37 age- and sex-matched healthy controls. For seed regions with significant structural changes, whole-brain functional connectivity (FC) was examined in a sub-cohort of N = 22 bvFTD and N = 22 matched control subjects to detect complementary alterations in brain network organization. To explore the functional significance of the observed structural and functional deviations, correlations with clinical and neuropsychological outcomes were tested where available. Significantly decreased CTh was observed in the bvFTD group in caudal middle frontal gyrus, left pars opercularis, bilateral superior frontal and bilateral middle temporal gyrus along with subcortical volume reductions in bilateral basal ganglia, thalamus, hippocampus, and amygdala. Resting-state functional magnetic resonance imaging showed decreased FC in bvFTD between: dorsal striatum and left caudal middle frontal gyrus; putamen and fronto-parietal regions; pallidum and cerebellum. Conversely, bvFTD showed increased FC between: left middle temporal gyrus and paracingulate gyrus; caudate nucleus and insula; amygdala and parahippocampal gyrus. Additionally, cortical thickness in caudal, lateral and superior frontal regions as well as caudate nucleus volume correlated negatively with apathy severity scores of the Neuropsychiatry Inventory Questionnaire. In conclusion, multimodal structural and functional imaging indicates that fronto-striatal regions have a considerable influence on the severity of apathy in bvFTD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frontotemporal dementia (FTD) is the second most common form of dementia in early age (< 65) (Onyike & Diehl-Schmid, 2013), clinically characterized by behavioral impairment, executive dysfunction and language deterioration. Phenotypes with predominant behavioral impairments and primary executive dysfunction are classified as behavioral variant FTD (bvFTD), while patients with language impairment are classified as primary progressive aphasia (Neary et al., 1998). 50–70% of the FTD patients are diagnosed as bvFTD (Johnson et al., 2005).
Studies have assessed the neuroanatomical underpinnings of apathy and disinhibition in bvFTD (Lansdall et al., 2017; Kumfor et al., 2018; Wei et al., 2020; Sheelakumari et al., 2020). Widespread volumetric changes were reported in frontal, temporal, and limbic areas along with cortical thickness (CTh) reductions in frontal, temporal and insular regions (Möller et al., 2016). Functional changes were studied using resting-state functional magnetic resonance imaging (rsfMRI), providing measures of spontaneous brain activity and functional connectivity (FC) (Azeez & Biswal, 2017). Decreased FC in bvFTD was reported within/between lateral prefrontal, basal ganglia, insular, hippocampal and amygdalar regions as well as salience and default mode networks (Ferreira et al., 2022; Kamalian et al., 2022). Conversely, increased FC was reported within the default mode network (Filippi et al., 2013; Whitwell et al., 2011). A relationship between altered mind-wandering capacity and structural / functional integrity of default and frontoparietal networks was reported in bvFTD using a multimodal approach (O’Callaghan et al., 2019). Another multimodal study reported that cortical thinning in temporal and orbitofrontal regions could predict clinical diagnosis of bvFTD (Canu et al., 2017). However, no study has as yet assessed structural alterations to determine seeds regions for analysis of FC and their relationship with neuropsychiatric symptoms within the same bvFTD cohort. Therefore, this study implemented a multimodal approach that first assessed surface-based CTh and subcortical volume changes and, second, examined “seed-to-whole brain” FC from brain areas showing structural alterations in bvFTD patients compared to matched HCs. We hypothesized structural changes in dorsolateral, middle frontal and temporal regions and associated reduced FC linked with behavioral symptoms in bvFTD.
Methods
Seventy-nine FTD patients were recruited in different clinics affiliated to the multicenter DESCRIBE (DZNE Clinical Register Study of Neurodegenerative Disorders) study at the German Center of Neurodegenerative Diseases (DZNE e.V.). Thirty-seven were diagnosed as bvFTD (Rascovsky et al., 2011) (age (mean ± SD) = 63.81 ± 11.32; sex (M:F) = 21:16) and included in the study. Five patients showed C9orf72 mutations, 1 had VCP and 1 FUS mutations. Three individuals had no DNA samples and the remaining 27 patients did not exhibit any mutations. Diagnostic criteria for bvFTD were presence of any three of clinically characterizing features like apathy/inertia, loss of sympathy, perseverative/compulsive behaviors, hyperorality, disinhibition and executive dysfunction (Rascovsky et al., 2011). The control group consisted of 37 age- (64.78 ± 7.63) and sex- (M: F = 22:15) matched healthy controls (HC).
Statements on Ethics and the Declaration of Helsinki are given in the Supplement, as well as inclusion and exclusion criteria and clinical, behavioral, neuropsychological measures. MRI data acquisition, analysis and statistical analysis is also mentioned in the Supplement.
Results
Demographic, clinical and behavioral variables (Table 1)
Among the demographic variables, age and sex were comparable between groups while education was significantly higher in HC (t(72) = 2.72; p = 0.008). The bvFTD patients showed significantly lower scores for MMSE (t(69)=-5.83; p < 0.001) and high scores on the NPI apathy scale (2.42 ± 0.68). Response inhibition in section B of the Hayling Sentence Completion Test (HSCT) was impaired, as indicated by fewer correct (t(38)=-4.28; p < 0.001) and more automated responses (category A errors: (t(38) = 4.53; p < 0.001). Moreover, short-term memory span (digit span forward: t(51)=-2.42; p < 0.021) and working memory (digit span backward: t(51)=-2.29; p < 0.031) were reduced.
For the demographic data of sub cohort of 22 bvFTD with complementary resting fMRI data and 22 matched controls see the supplementary Table 1.
Cortical thickness
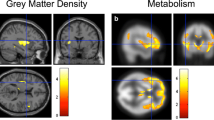
A significant bilateral cortical thinning was observed in bvFTD patient group in the left hemisphere, affecting caudal middle frontal gyrus (CMFG), middle temporal gyrus (MTG), pars opercularis and superior frontal gyrus (SFG). In the right hemisphere, only MTG and SFG showed significant cortical thinning (Fig. 1 part 1.1).
Display of structural changes in 37 bvFTD as compared to 37 healthy controls: 1.1) Decreased cortical thickness in 37 bvFTD (blue color) as well as Changes in FC when considered regions exhibiting cortical thinning and sub cortical volume loss as seeds in 22 bvFTD compared to 22 HC; 1.2a) Decreased FC with respect to left caudal middle frontal gyrus and; 1.2b) Increased FC with respect to left middle temporal gyrus; 1.3a) decreased FC with respect to putamen; 1.3b) decreased FC with respect to pallidum; and 1.3c) increased FC with respect to caudate nucleus; 1.3d) increased FC with respect to amygdala; and 1.3e) increased FC with respect to hippocampus. Here, decrease in FC is presented with blue color while increase with red-yellow. FC = functional connectivity; L = left; R = right
Subcortical volume
A general linear model implementing age, sex, education, total intracranial volume and scanner site as nuisance variables showed significant bilateral volume losses in thalamus, caudate nucleus, putamen, pallidum, amygdala, hippocampus and nucleus accumbens in bvFTD (Supplementary Table 2).
Cortical seeds-to-whole-brain FC
Among all cortically thinned regions only left CMFG and left MTG showed significant FC changes in bvFTD. Furthermore, left caudal middle frontal gyrus showed decreased FC with dorsal striatum, anterior thalamus and cerebellar regions (Fig. 1 part 1.2a). Conversely, left MTG showed increased FC with paracingulate gyrus (Fig. 1 part 1.2b).
Subcortical seeds-to-whole-brain FC
For subcortical regions, decreased and increased FC was observed in bvFTD: Putamen showed decreased FC with cingulate, medial, lateral frontal and parietal cortex (Fig. 1 part 1.3a) and pallidum with medial and lateral cerebellar lobes (Fig. 1 part 1.3b). Conversely, caudate nucleus showed increased FC with right insula and inferior frontal gyrus (Fig. 1 part 1.3c). Amygdala and hippocampus showed increased FC with right parahippocampal gyrus, temporal pole and central opercular cortex (Fig. 1 parts 1.3d and 1.3e, respectively). No FC changes occurred in networks related to thalamus and nucleus accumbens seeds.
Clinical correlations
Clinical correlations were assessed in 37 bvFTD patients, showing CDR-SOB to correlate positively with apathy subscores of the NPI-Q (r = 0.69, p < 0.001) and negatively with MMSE (r= -0.54, p = 0.011) in bvFTD.
Clinico-radiological correlations
Clinico-radiological correlations between structural and clinical and neuropsychological scores were measured in 37 bvFTD. CDR-SOB scores correlated negatively with CTh measures in left MTG (r= -0.47; p = 0.004), left SFG (r= -0.52; p = 0.001), left CMFG (r= -0.47; p = 0.004), and right SFG (r= -0.62; p < 0.001). Correlations with NPI-Q sub-scores for apathy severity showed negative correlations with CTh in left pars opercularis (r=-0.54; p = 0.011), left CMFG (r=-0.54; p = 0.011), and right SFG (r=-0.57; p = 0.007). Moreover, in subcortical regions, only bilateral caudate nuclei showed trend negative correlations (r=-0.57, p = 0.007) with apathy severity of NPI-Q.
Discussion
This multimodal MRI study investigated structural and functional changes and their association with neuropsychiatric symptoms, notably apathy, disinhibition and executive dysfunction in patients with bvFTD. Significant cortical thinning was found in frontal and temporal regions in bvFTD, along with subcortical volumetric reductions in all seven tested regions. Assessment of FC in seed regions obtained from structural analyses helped identifying functional changes associated with underlying structural loss: Decreased FC was found between left CMFG and anterior caudate nucleus and increased FC between left MTG and paracingulate gyrus. Similarly, subcortical seed regions also showed both decreased and increased FC in bvFTD. Putamen and pallidum showed decreased FC with fronto-parietal and cerebellar areas, respectively, while caudate nucleus, amygdala and hippocampus demonstrated increased FC with insula, inferior frontal and parahippocampus gyrus. Additionally, correlation analyses between clinical, behavioral and structural measures suggest that cortical thinning in frontal regions and volume loss in caudate nucleus relate with apathy severity in bvFTD.
Previous studies reported lateral and dorsal frontal areas to be predominantly linked with apathy in bvFTD (Moretti & Signori, 2016; Ducharme et al., 2018; Sheelakumari et al., 2020; Jenkins et al., 2022). We found cortical thinning in left CMFG, left pars opercularis, and right SFG to correlate with apathy severity. Middle frontal areas are involved in motivated behaviors (Kouneiher et al., 2009) whereas lateral frontal areas are more involved in cognitive control (Bahlmann et al., 2015). Thus, we speculate that the structural reductions cause amotivated behavior and reduced cognitive control (shown by higher scores for HSCT error of category A), possibly leading to apathetic behavior in bvFTD.
Affection of the basal ganglia also seems to play a distinctive role for apathy (Jenkins et al., 2022). Basal ganglia atrophy, especially of caudate nucleus, putamen and pallidum, has been reported in bvFTD (Bertoux et al., 2015; Macfarlane et al., 2015) and was reported to be relevant for apathy in bvFTD (Jenkins et al., 2022). Our observations of caudate atrophy in relation with increased apathy severity gives further support to the striatal role for apathy in bvFTD (Bertoux et al., 2015; Kumfor et al., 2018).
Beyond the independent role of the frontal cortex and the basal ganglia for apathy outlined above, fronto-striatal circuit impairment has been linked to apathy in elderly individuals (Hamada et al., 2021). The novelty of the current study is the demonstration of fronto-striatal circuit impairment, indexed as altered FC, in bvFTD. Notably, decreased FC between left CMFG and dorsal striatum was a principal finding, suggesting perturbation of fronto-striatal networks in bvFTD. Moreover, NPI-Q sub-scores for apathy severity were inversely correlated with cortical thickness in frontal and volume loss in striatal regions, strengthening the pathophysiological relevance of our multimodal approach in bvFTD.
In conclusion, the presented data indicate that structural alterations in frontal and striatal regions lead to disintegration of fronto-striatal networks in bvFTD and contribute to apathy severity, a core clinical symptom of the disease. Meanwhile, we acknowledge the small sample size and some missing behavioral measures. Future multimodal studies with bigger sample sizes and complete data sets warrant further validation of our observations.
Data availability
Due to limits on data distribution and usage imposed by patient agreement, the data used in the current investigation are not publicly accessible, although they may be obtained upon justifiable request from the DESCRIBE, DELCODE, and DANCER study consortia.
Abbreviations
- bvFTD:
-
Behavioral variant frontotemporal dementia
- CDR-SOB:
-
Clinical Dementia Rating Scale - Sum Of Boxes
- FTD:
-
Frontotemporal dementia
- FC:
-
Functional connectivity
- HSCT:
-
Hayling Sentence Completion Test
- MRI:
-
Magnetic resonance imaging
- MMSE:
-
Mini Mental Screening Examination
- NPI-Q:
-
Neuropsychiatric Inventory Questionnaire
- nc:
-
Number count
- rsfMRI:
-
Resting-state functional magnetic resonance imaging
References
Azeez, A. K., & Biswal, B. B. (2017). A review of resting-state analysis methods. Neuroimaging Clinics of North America, 27(4), 581–592. https://doi.org/10.1016/j.nic.2017.06.001.
Bahlmann, J., Aarts, E., & D’Esposito, M. (2015). Influence of motivation on control hierarchy in the human frontal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(7), 3207–3217. https://doi.org/10.1523/JNEUROSCI.2389-14.2015.
Bertoux, M., O’Callaghan, C., Flanagan, E., Hodges, J. R., & Hornberger, M. (2015). Fronto-Striatal atrophy in behavioral variant Frontotemporal Dementia and Alzheimer’s Disease. Frontiers in Neurology, 6, 147. https://doi.org/10.3389/fneur.2015.00147.
Canu, E., Agosta, F., Mandic-Stojmenovic, G., Stojković, T., Stefanova, E., Inuggi, A., Imperiale, F., Copetti, M., Kostic, V. S., & Filippi, M. (2017). Multiparametric MRI to distinguish early onset Alzheimer’s Disease and behavioural variant of frontotemporal Dementia. NeuroImage Clinical, 15, 428–438. https://doi.org/10.1016/j.nicl.2017.05.018.
Ducharme, S., Price, B. H., & Dickerson, B. C. (2018). Apathy: A neurocircuitry model based on frontotemporal Dementia. Journal of Neurology Neurosurgery and Psychiatry, 89(4), 389–396. https://doi.org/10.1136/jnnp-2017-316277.
Ferreira, L. K., Lindberg, O., Santillo, A. F., & Wahlund, L. O. (2022). Functional connectivity in behavioral variant frontotemporal Dementia. Brain and Behavior, 12(12), e2790. https://doi.org/10.1002/brb3.2790.
Filippi, M., Agosta, F., Scola, E., Canu, E., Magnani, G., Marcone, A., Valsasina, P., Caso, F., Copetti, M., Comi, G., Cappa, S. F., & Falini, A. (2013). Functional network connectivity in the behavioral variant of frontotemporal Dementia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(9), 2389–2401. https://doi.org/10.1016/j.cortex.2012.09.017.
Hamada, C., Kawagoe, T., Takamura, M., Nagai, A., Yamaguchi, S., & Onoda, K. (2021). Altered resting-state functional connectivity of the frontal-striatal circuit in elderly with apathy. PloS One, 16(12), e0261334. https://doi.org/10.1371/journal.pone.0261334.
Jenkins, L. M., Wang, L., Rosen, H., & Weintraub, S. (2022). A transdiagnostic review of neuroimaging studies of apathy and disinhibition in Dementia. Brain: A Journal of Neurology, 145(6), 1886–1905. https://doi.org/10.1093/brain/awac133.
Johnson, J. K., Diehl, J., Mendez, M. F., Neuhaus, J., Shapira, J. S., Forman, M., Chute, D. J., Roberson, E. D., Pace-Savitsky, C., Neumann, M., Chow, T. W., Rosen, H. J., Forstl, H., Kurz, A., & Miller, B. L. (2005). Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Archives of Neurology, 62(6), 925–930. https://doi.org/10.1001/archneur.62.6.925.
Kamalian, A., Khodadadifar, T., Saberi, A., Masoudi, M., Camilleri, J. A., Eickhoff, C. R., Zarei, M., Pasquini, L., Laird, A. R., Fox, P. T., Eickhoff, S. B., & Tahmasian, M. (2022). Convergent regional brain abnormalities in behavioral variant frontotemporal Dementia: A neuroimaging meta-analysis of 73 studies. Alzheimer’s & Dementia (Amsterdam Netherlands), 14(1), e12318. https://doi.org/10.1002/dad2.12318.
Kouneiher, F., Charron, S., & Koechlin, E. (2009). Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience, 12(7), 939–945. https://doi.org/10.1038/nn.2321.
Kumfor, F., Zhen, A., Hodges, J. R., Piguet, O., & Irish, M. (2018). Apathy in Alzheimer’s Disease and frontotemporal Dementia: Distinct clinical profiles and neural correlates. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 103, 350–359. https://doi.org/10.1016/j.cortex.2018.03.019.
Lansdall, C. J., Coyle-Gilchrist, I. T. S., Jones, P. S., Rodríguez, V., Wilcox, P., Wehmann, A., Dick, E., Robbins, K. M., T. W., & Rowe, J. B. (2017). Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain: A Journal of Neurology, 140(6), 1792–1807. https://doi.org/10.1093/brain/awx101.
Macfarlane, M. D., Jakabek, D., Walterfang, M., Vestberg, S., Velakoulis, D., Wilkes, F. A., Nilsson, C., van Westen, D., Looi, J. C. L., & Santillo, A. F. (2015). Striatal atrophy in the behavioural variant of Frontotemporal Dementia: Correlation with diagnosis, negative symptoms and Disease Severity. PloS One, 10(6), e0129692. https://doi.org/10.1371/journal.pone.0129692.
Möller, C., Hafkemeijer, A., Pijnenburg, Y. A. L., Rombouts, S. A. R. B., van der Grond, J., Dopper, E., van Swieten, J., Versteeg, A., Steenwijk, M. D., Barkhof, F., Scheltens, P., Vrenken, H., & van der Flier, W. M. (2016). Different patterns of cortical gray matter loss over time in behavioral variant frontotemporal Dementia and Alzheimer’s Disease. Neurobiology of Aging, 38, 21–31. https://doi.org/10.1016/j.neurobiolaging.2015.10.020.
Moretti, R., & Signori, R. (2016). Neural correlates for apathy: Frontal-prefrontal and parietal cortical- subcortical circuits. Frontiers in Aging Neuroscience, 8, 289. https://doi.org/10.3389/fnagi.2016.00289.
Neary, D., Snowden, J. S., Gustafson, L., Passant, U., Stuss, D., Black, S., Freedman, M., Kertesz, A., Robert, P. H., Albert, M., Boone, K., Miller, B. L., Cummings, J., & Benson, D. F. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554. https://doi.org/10.1212/wnl.51.6.1546.
O’Callaghan, C., Shine, J. M., Hodges, J. R., Andrews-Hanna, J. R., & Irish, M. (2019). Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America, 116(8), 3316–3321. https://doi.org/10.1073/pnas.1818523116.
Onyike, C. U., & Diehl-Schmid, J. (2013). The epidemiology of frontotemporal Dementia. International Review of Psychiatry (Abingdon England), 25(2), 130–137. https://doi.org/10.3109/09540261.2013.776523.
Rascovsky, K., Hodges, J. R., Knopman, D., Mendez, M. F., Kramer, J. H., Neuhaus, J., van Swieten, J. C., Seelaar, H., Dopper, E. G. P., Onyike, C. U., Hillis, A. E., Josephs, K. A., Boeve, B. F., Kertesz, A., Seeley, W. W., Rankin, K. P., Johnson, J. K., Gorno-Tempini, M. L., Rosen, H., & Miller, B. L. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal Dementia. Brain: A Journal of Neurology, 134(Pt 9), 2456–2477. https://doi.org/10.1093/brain/awr179.
Sheelakumari, R., Bineesh, C., Varghese, T., Kesavadas, C., Verghese, J., & Mathuranath, P. S. (2020). Neuroanatomical correlates of apathy and disinhibition in behavioural variant frontotemporal Dementia. Brain Imaging and Behavior, 14(5), 2004–2011. https://doi.org/10.1007/s11682-019-00150-3.
Wei, G., Irish, M., Hodges, J. R., Piguet, O., & Kumfor, F. (2020). Disease-specific profiles of apathy in Alzheimer’s Disease and behavioural-variant frontotemporal Dementia differ across the Disease course. Journal of Neurology, 267(4), 1086–1096. https://doi.org/10.1007/s00415-019-09679-1.
Whitwell, J. L., Josephs, K. A., Avula, R., Tosakulwong, N., Weigand, S. D., Senjem, M. L., Vemuri, P., Jones, D. T., Gunter, J. L., Baker, M., Wszolek, Z. K., Knopman, D. S., Rademakers, R., Petersen, R. C., Boeve, B. F., & Jack, C. R. (2011). Altered functional connectivity in asymptomatic MAPT subjects: A comparison to bvFTD. Neurology, 77(9), 866–874. https://doi.org/10.1212/WNL.0b013e31822c61f2.
Acknowledgements
The authors thank all patients with FTD, their caregivers and healthy control individuals who participated in this multicenter study. The authors also thank to the individuals involved in clinical, behavioral and MRI, data acquisition and data management at various centers of German Center for Neurodegenerative Diseases, Max-Delbrück-Centrum für Molekulare Medizin in der Helmholtz-Gemeinschaft (MDC), Freie Universität Berlin Center for Cognitive Neuroscience, Berlin (CCNB), Bernstein Center für Computional Neuroscience, Berlin, and Institut für Klinische Radiologie, Klinikum der Universität, München. The authors would like to extend their acknowledgments to Marcel Daamen, a valued colleague, for his dedicated and meticulous contributions to the revision of this manuscript.
Funding
The study was funded by German Center for Neurodegenerative Diseases (Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE)), reference numbers BN006 (DESCRIBE), BN026 (DANCER) and BN012 (DELCODE). DESCRIBE was additionally supported by the Nomis Foundation”.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The BvFTD study was conceptualised and analysed by Neeraj Upadhyay. Data and resources were largely provided by DZNE sites. Individuals involved in the data acquisition process were Ingo Frommann, Klaus Fliessbach, Benjamin Bender, Wenzel Glanz, Katharina Buerger, Daniel Janowitz, Adrian Danek, Ingo Kilimann, Matthis Synofzik, Carlo Wilke, Lukas Preis, Josef Priller and Eike Jakob Spruth. Annika Spottke, Anja Schneider, Hauke R. Heekeren, Michael Ewers, Emrah Düzel, John Dylan Haynes, Johannes Levin, Oliver Peters, Stefan Teipel, and Frank Jessen were involve in designing and executing the DESCRIBE-FTD study. Daniel Hoffmann the data and involved in management. Quality assurance of imaging data was done by Laura Dobisch and Neeraj Upadhyay. Responsible for drafting the manuscript were Neeraj Upadhyay and Henning Boecker. Statistical analysis and interpretation of the data was carried out by Neeraj Upadhyay. Neeraj Upadhyay, Henning Boecker, Tommaso Ballarini and Ingo Frommann edited the manuscript. All authors critically reviewed the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the local ethics committees of the participating sites under coordination of the University Hospital Bonn (Medical Faculty of Rheinische Friedrich-Wilhelms-Universität Bonn: Lfd. Nr. 311/14 (DESCRIBE), 117/13 (DELCODE), and 312/14 (DANCER)), and in accordance with national legislation and the Declaration of Helsinki.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Upadhyay, N., Spottke, A., Schneider, A. et al. Fronto-striatal alterations correlate with apathy severity in behavioral variant frontotemporal dementia. Brain Imaging and Behavior 18, 66–72 (2024). https://doi.org/10.1007/s11682-023-00812-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-023-00812-3