Abstract
Recent neuroimaging studies have reported alterations in brain activation during cognitive tasks in cancer patients who have undergone chemotherapy treatment. However, the location of these altered brain activation patterns after chemotherapy varies considerably across studies. The aim of the present meta-analysis was to quantitatively synthesise this body of evidence using Activation Likelihood Estimation to identify reliable regions of altered brain activation in cancer patients treated with chemotherapy, compared to healthy controls and no chemotherapy controls. Our systematic search identified 12 studies that adopted task-related fMRI on non-central nervous system cancer patients who received chemotherapy relative to controls. All studies were included in the analyses and were grouped into four contrasts. Cancer patients treated with chemotherapy showed reduced activation in the left superior parietal lobe/precuneus (family-wise error corrected p < .05) compared to no chemotherapy controls. No significant clusters were found in three of our contrasts. The majority of studies did not support an association between altered brain activation and cognitive performance after chemotherapy. Findings point towards a possible chemotherapy-induced alteration, which could inform targeted treatment strategies. With continued work in this field using homogenous task-related protocols and cancer populations, fMRI may be used as a biomarker of cognitive deficits in the future.
Similar content being viewed by others
Introduction
Cancer is a major cause of death and illness worldwide, with an estimated 19.3 million new cases and 10 million deaths in 2020 (Sung et al., 2021). While chemotherapy has improved cancer survival rates, it is often associated with adverse treatment-related side-effects, including cognitive deficits (Janelsins et al., 2014). Self-reported measures and computerised neuropsychological tests have identified cognitive deficits in cancer survivors who have undergone chemotherapy regimens, often dubbed “chemobrain” or “chemofog” (for a review, see Hutchinson et al., 2012). The most prominently affected domains in cognitive functioning include impairments in processing speed, memory and executive functions (Ahles et al., 2002; Jansen et al., 2011; Wefel et al., 2010). These symptoms are prevalent across all types of cancer, including those which manifest outside the central nervous system (CNS). Cognitive deficits can have pervasive impacts on cancer survivors’ quality of life, interfering with their capacity to accomplish daily tasks (e.g., cooking and driving), as well as their interpersonal relationships and occupational performance (Boykoff et al., 2009; Henderson et al., 2019; Myers, 2012). Therefore, it is important to examine the biological underpinnings of cognitive deficits following chemotherapy to inform treatment strategies.
Over the past two decades, functional magnetic resonance imaging (fMRI) studies have yielded novel insights into the neural substrates of cognitive deficits in cancer patients by investigating aspects of brain function (for a review, see Sousa et al., 2020). Task-related fMRI has been utilised to detect neural abnormalities in cancer patients treated with chemotherapy (CTx+) by comparing brain activation patterns during cognitive tasks with healthy controls (HC) and/or cancer patients who received surgery or adjuvant treatments but not chemotherapy (CTx−; no chemotherapy controls). Utilising this MRI technique for the first time, a case study presented in Ferguson et al. (2007) compared monozygotic twins, reporting increased frontal and parietal activation during a working memory task in a twin treated with chemotherapy compared to the healthy twin. Since this initial contribution, several cohort task-related fMRI studies have been undertaken. In one of the first group studies, Kesler et al. (2009) reported both increased activation in multiple brain regions during a memory recall task and decreased activation in the prefrontal cortex during a memory encoding task in CTx+ patients relative to HC. More recent studies have observed similar findings to Ferguson et al. (2007), with increased activation found in frontal regions during working memory and episodic memory tasks in CTx+ patients compared to controls (McDonald et al., 2012; Pergolizzi et al., 2019). Conversely, other studies have shown reduced activation in frontal and parietal regions (Correa et al., 2017; López Zunini et al., 2013; Stouten-Kemperman et al., 2015) and subcortical regions (e.g., hippocampus and amygdala; de Ruiter et al., 2011; Vardy et al., 2019; Wang et al., 2016) in CTx+ patients relative to controls. Despite these notable findings, the localisation and direction of altered brain activation patterns are mixed across studies, rendering interpretation difficult.
Among popular theories, brain regions of decreased activation in CTx+ patients compared to controls, have been interpreted as the result of chemotherapy-induced damage (Correa et al., 2017; Kesler et al., 2011). Animal studies have demonstrated that non-CNS chemotherapeutic agents can have neurotoxic effects on the structure and function of normal cells in the nervous system (Briones & Woods, 2011; Christie et al., 2012; Yan et al., 2015). Contrarily, increased brain activation in CTx+ patients compared to controls, has been suggested as a compensatory mechanism for dysfunction in task-relevant brain regions, with the recruitment of additional brain regions required to reach the same level of performance (Ferguson et al., 2007; McDonald et al., 2012; Menning et al., 2017). With the identification of consistent altered brain activation patterns in CTx+ patients, present understandings may be strengthened.
To date, a number of scoping and systematic reviews have performed a qualitative comparison on brain activation patterns in cancer survivors across several studies, highlighting diffuse alterations in frontal and parietal regions after chemotherapy (Andryszak et al., 2017; de Ruiter & Schagen, 2013; Li & Caeyenberghs, 2018; Pomykala et al., 2013; Scherling & Smith, 2013; Simó et al., 2013; Sousa et al., 2020). Aside from these reviews, only one quantitative study (Kesler, 2014) has been published so far. In this study by Kesler (2014) a meta-analytical technique known as Activation Likelihood Estimation (ALE) was used to synthesise data across six fMRI studies (Conroy, McDonald, Smith, et al., 2013a; de Ruiter et al., 2011; Kesler et al., 2009; Kesler et al., 2011; López Zunini et al., 2013; McDonald et al., 2012), revealing consistently reduced activation in brain regions involved in the default mode network (e.g., the precuneus and medial frontal gyrus). However, this study was limited by not reporting the group contrasts and methodological procedures used in their ALE analysis.
In the present study, we will integrate the most-recent fMRI data to identify robust patterns across studies, utilising ALE. The ALE approach has successfully been employed to map neural correlates of symptoms in a wide array of neurological and psychiatric populations, including patients with Parkinson’s disease (Santangelo et al., 2019), traumatic brain injury (Cook et al., 2020) and major depressive disorders (Zhang et al., 2016). During fMRI, neural locations of peak activation evoked by performance on functional tasks are recorded in stereotaxic coordinates (Fuelscher et al., 2018). An ALE meta-analysis aggregates these significant activation coordinates from multiple neuroimaging studies to identify voxel-wise regions of spatial convergence (Eickhoff et al., 2009; Eickhoff et al., 2012). The resulting map incorporates commonly activated neuroanatomical regions across individual studies (Eickhoff et al., 2012). In other words, ALE enables a way of determining how likely a neural location is to be involved in a symptom based on several studies (Eickhoff et al., 2012; Fuelscher et al., 2018). Overall, ALE offers a powerful method for aggregating data from neuroimaging studies as it attempts to minimise study-specific noise of small sample sizes and various experimental tasks (Eickhoff et al., 2009; Eickhoff et al., 2012). An ALE analysis will therefore provide much needed clarification into the potential neural mechanisms underlying cognitive deficits in cancer patients after chemotherapy.
Aims and hypotheses
The aims of the present study are twofold. The primary aim is to conduct an ALE meta-analysis to identify reliable regions of altered brain activation in CTx+ patients across several task-related fMRI studies. Specifically, we will examine whether there are differences in brain activation patterns during cognitive tasks between non-CNS CTx+ patients, compared to HC and CTx− patients. It is hypothesised that ALE will reveal consistent alterations in brain activation (i.e., increased activation in frontal and parietal regions and decreased activation in subcortical regions) in CTx+ patients compared to control groups. Our secondary aim is to explore the association between altered brain activation and cognitive performance (i.e., using performance scores from a cognitive task performed as part of the behavioral test battery) in CTx+ patients. This may inform whether regions of altered brain activation patterns are reliable biomarkers of cognitive deficits experienced by cancer survivors. Mixed results have been found in the literature with some studies reporting a positive association (i.e., decreased brain activation associated with worse cognitive task performance; de Ruiter et al., 2011) and other studies revealing no significant association (Correa et al., 2017; Wang et al., 2016). It is expected that reduced brain activation will be related to diminished performance on cognitive tasks in CTx+ patients.
Methods
Systematic search
A systematic search of peer-reviewed literature published up to 30 July 2020 was conducted. Embase, PsycInfo and MEDLINE Complete databases were searched. The search string involved a combination of keywords including “fMRI”, “cancer”, “chemotherapy” and “cognitive dysfunction”, and their synonyms, based on a recent systematic review (Li & Caeyenberghs, 2018). See Appendix 1 Table 3 for the full search syntax. No publication date or language limiters were applied. Reference sections of eligible studies were inspected to identify additional articles of interest.
Studies were included in the analysis if they met the following criteria: (1) utilised task-related fMRI as the main neuroimaging modality; (2) participants performed a cognitive task during scanning; (3) reported coordinates of activation foci in either Montreal Neurological Institute (MNI) or Talairach reference space; (4) reported group comparisons between cancer patients treated with chemotherapy compared with healthy controls and/or no chemotherapy controls. Studies with only restricted regions of interest (ROI) analyses were excluded to ensure that the likelihood of activation was equal across the brain (Eickhoff et al., 2009; Muller et al., 2018). Articles that investigated cancer of the brain or central nervous system (CNS) were also omitted as neurosurgery and CNS-directed chemotherapy are known to induce neurocognitive impairments or brain alterations (Li & Caeyenberghs, 2018). Studies conducted in paediatric cancer populations (age < 18 years) and animal models were removed. Finally, conference abstracts, case studies and systematic reviews were excluded.
Data extraction
We extracted and summarised data on clinical population characteristics from each study, including the age and gender of participants, cancer type, time since treatment, chemotherapy regimens and adjuvant treatments (see Appendix 2 Table 4). The steps for neuroimaging analyses of each study are detailed in Table 1, including group contrasts, sample size, activation foci, brain template, statistical thresholding and fMRI paradigm used. Finally, data was extracted from selected studies which performed correlation analyses between regions of altered brain activation patterns and cognitive performance in the chemotherapy-treated group, including the significance of the relationship, correlation coefficients, and the involved brain regions (Table 1).
The parameters for an ALE analysis (i.e., sample size and activation foci) were manually imported into text files according to the type of group contrast and the direction of the contrast. Coordinates that were reported in Talairach space were transformed into MNI coordinates using the BioImage Suite tool (Lacadie et al., 2008). In studies that reported foci from two different paradigms (Correa et al., 2017; de Ruiter et al., 2011; Kesler et al., 2009; Kesler et al., 2011; Stouten-Kemperman et al., 2015), the foci were included as one experiment. However, for the study of Pergolizzi et al. (2019), results were imported as two experiments to account for the different number of participants that completed each paradigm. For longitudinal studies (López Zunini et al., 2013; McDonald et al., 2012; Menning et al., 2017; Pergolizzi et al., 2019), data was extracted from the last follow-up time-point. In studies that examined two cohorts of chemotherapy-treated patients, coordinates were selected from the groups of patients who received standard-dose chemotherapy (Stouten-Kemperman et al., 2015) and patients who reported cognitive symptoms (Vardy et al., 2019).
Activation Likelihood Estimation
To examine the first research question, we conducted four independent ALE analyses using Ginger ALE’s (version 2.3.6; http://brainmap.org) random effects algorithm (Eickhoff et al., 2009; Turkeltaub et al., 2012). The ALE technique aggregates statistically significant foci reported from multiple neuroimaging studies. In other words, contrasts of ALE meta-analyses only include studies that have reported coordinates of brain regions that revealed significant group differences in brain activation (i.e., significant increases or decreases in activation between CTx+ patients and controls). This allows us to identify the brain regions which are most commonly implicated in cognitive symptoms in cancer patients across studies (Acar et al., 2018; Eickhoff et al., 2009). Figure 1 depicts a step-by-step overview of the procedure. Firstly, peak coordinates reported from each neuroimaging study are individually mapped onto a standardised brain. Secondly, to account for spatial uncertainty, Gaussian kernels are applied around the foci, whereby the diameter of the kernel is determined by the study sample size (e.g., smaller studies have more spatial uncertainty and therefore we apply larger kernels; Acar et al., 2018). This process results in a series of modelled activation (MA) maps. Next, an ALE map is computed by calculating the union of these MA maps. In the final step, the ALE map is thresholded by testing the activation likelihood values at each voxel against a null distribution of random spatial associations among studies, to establish at which locations the convergence of foci is greater than can be expected by chance (Eickhoff et al., 2012).
Overview of the ALE meta-analysis procedure. Note. R = right hemisphere; L = left hemisphere. Orange, green and yellow colours indicate activation foci obtained from the different studies. Brain surface models were created using the NeuroMarvl tool (https://immersive.erc.monash.edu/neuromarvl/)
We corrected for multiple comparisons utilising a cluster-level family-wise error (FWE) threshold of p < .05, with 1000 permutations, following an initial cluster-forming threshold of uncorrected p < .001. This thresholding procedure provides an appropriate balance between sensitivity and specificity and is in line with recommendations from Eickhoff et al. (2012). The resulting maps were overlaid onto a standard anatomical template in MNI space using Mango (http://ric.uthscsa.edu/mango/) to visualise two-dimensional slices and MRIcroGL (https://www.nitrc.org/projects/mricrogl) to render a three-dimensional image.
In the first ALE, we tested for decreased brain activation in cancer patients treated with chemotherapy compared to healthy controls (CTx+ < HC). The second ALE examined increased brain activation in cancer patients treated with chemotherapy relative to healthy controls (CTx+ > HC). The third ALE tested for decreased brain activation in cancer patients treated with chemotherapy compared to no chemotherapy controls (CTx+ < CTx−). In the fourth ALE, we looked for increased brain activation in cancer patients treated with chemotherapy compared to no chemotherapy controls (CTx+ > CTx−). Finally, for contrasts that reported no significant clusters, we conducted an exploratory analysis, using an uncorrected p < .001 and minimum cluster sizes of 50mm3.
Behavioral metrics
To investigate the second research question, correlation analyses from each study were reviewed to observe overall data trends. Next, we calculated effect sizes for main findings where information was available (i.e., R2 = squared correlation coefficient). Following Cohen’s (1988) conventions, .01 was regarded as a weak relationship, .09 a moderate relationship, and .25 a strong relationship.
Results
Search results
The search of databases identified a total of 446 studies, of which 21 were assessed for eligibility at full text (see Fig. 2 for the PRISMA flow diagram). We excluded four studies, which conducted a region of interest (ROI) analysis (executive control subnetwork; Askren et al., 2014; Hosseini & Kesler, 2014; Jung et al., 2017; multitasking subnetwork; Deprez et al., 2014). We also omitted one case study (Ferguson et al., 2007), one resting-state fMRI study (Apple et al., 2018) and one study that used a facial expression fMRI task (Stouten-Kemperman et al., 2018). Moreover, one study was removed as we were unable to verify the brain template (Kam et al., 2016). Another study was excluded as the peak coordinates were not reported (Conroy, McDonald, Ahles, et al., 2013b). This resulted in a final set of 12 task-related fMRI studies published between 2009 and 2019, included in the current study. Of these 12 studies, eight reported significant results for the CTx+ < HC contrast (21 foci), three for the CTx+ > HC contrast (12 foci), four for the CTx+ < CTx− contrast (26 foci) and three for the CTx+ > CTx− contrast (13 foci; as can be seen in Fig. 2).
PRISMA flow diagram of the study selection process, adapted from Moher et al. (2009). Note. The final analysis section of the flow diagram depicts the total number of studies reporting significant coordinates for each group contrast and the total number of coordinates extracted from those studies. CTx+ = cancer patients treated with chemotherapy; CTx− = no chemotherapy controls; HC = healthy controls; < = decreased brain activation; > = increased brain activation; ROI = regions of interest; n = number of studies
Study characteristics
Studies were conducted in the United States of America (n = 7), Netherlands (n = 3) and Canada (n = 2). Cancer populations investigated included breast (10, 83%), ovarian (1, 8%) and colorectal, Hodgkin’s lymphoma, leukaemia and melanoma (1, 8%) cancers. The selected studies involved cancer patients treated with chemotherapy (N = 240), healthy controls (N = 103) and no chemotherapy controls (N = 134), with an average age of 53.72 years (SD = 7.7). Two studies used both a healthy control group and no chemotherapy control group. Four studies used a no chemotherapy control group and six studies used only a healthy control group. Of note, no chemotherapy controls encompassed cancer patients who had undergone surgery and, in some cases, had also received endocrine and/or radiation therapy as part of their cancer treatment (see Appendix 2). Most cancer patients were treated with standard-dose chemotherapy regimens, which included a combination of chemotherapeutic agents, such as cyclophosphamide, cisplatin, carboplatin, docetaxel, doxorubicin, epirubicin, fluorouracil, paclitaxel, methotrexate, and thiotepa. The time between completion of chemotherapy treatment and fMRI scan, spanned one month to 13 years. Various fMRI paradigms were utilised to evaluate brain activation including a wide array of working memory, executive function, episodic or verbal memory and attention tasks. Full clinical population characteristics and neuroimaging details are summarised in Appendix 2 Table 4 and Table 1, respectively.
ALE meta-analyses
Alterations in cancer patients treated with chemotherapy compared to healthy controls.
No significant clusters were found between CTx+ patients and HC, using an exploratory uncorrected threshold (p < .001).
Alterations in cancer patients treated with chemotherapy compared to no chemotherapy controls
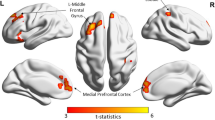
As can be seen in Fig. 3, CTx+ patients showed a pattern of decreased brain activation in the left superior parietal lobe/precuneus (Brodmann area 7) compared to CTx− patients (FWE corrected). Table 2 provides the MNI coordinates of significant functional brain activation. No significant results were found in the opposite direction (CTx+ > CTx−) using an uncorrected threshold.
Meta-analytical maps of decreased brain activation in cancer patients treated with chemotherapy compared to no chemotherapy controls. Note: Decreased brain activation in the chemotherapy-treated group was localised in the left superior parietal lobe/precuneus displayed on coronal (A), sagittal (B) and axial (C) planes and a rendered image (D). Right and left hemispheres of the brain are presented according to radiological conventions. Values indicate MNI coordinates (x, y, z). Image thresholded at p < .003 uncorrected, for visualisation purposes. The largest cluster on the axial plane, indicated by a red arrow, is the only cluster that survived family-wise error corrections
Correlation between functional brain alterations and cognition
Seven of 12 studies tested for correlations between altered brain activation patterns and cognitive performance in the CTx+ group (Conroy, McDonald, Smith, et al., 2013a; Correa et al., 2017; de Ruiter et al., 2011; Kesler et al., 2009; Kesler et al., 2011; Stouten-Kemperman et al., 2015; Wang et al., 2016). Five of these seven studies (71%) reported a non-significant association between functional brain alterations and cognitive performance. Only two of these seven studies revealed a statistically-significant association (de Ruiter et al., 2011; Stouten-Kemperman et al., 2015). Specifically, reduced activation in the dorsolateral prefrontal cortex and hippocampal regions were related to a decline in cognitive performance on executive function (R2 = 59%) and episodic memory (R2 = 62%) tasks, respectively (de Ruiter et al., 2011; Stouten-Kemperman et al., 2015). These effect sizes were calculated from de Ruiter et al. (2011) only, as limited information was provided in Stouten-Kemperman et al. (2015). Following Cohen’s (1988) conventions, these associations can be regarded as strong relationships.
Discussion
This study set out to map the common regions of altered brain activation patterns in non-CNS cancer patients who have undergone chemotherapy. By combining coordinates of peak activation from 240 cancer patients treated with chemotherapy (CTx+), 103 healthy controls (HC) and 134 no chemotherapy controls (CTx−), the present ALE meta-analysis is one of the first studies to quantitatively summarise the existing fMRI data. Our findings revealed decreased activation in the left precuneus located in the superior parietal lobe in CTx+ patients. No significant clusters were detected in three of our contrasts (i.e., CTx+ > CTx−, CTx+ < HC and CTx+ > HC). Furthermore, our results showed that the majority of studies did not provide evidence to suggest that altered brain activation following chemotherapy is related to performance on cognitive tasks. To this end, we will discuss a few methodological considerations for future task-related fMRI studies.
The role of the precuneus in cancer patients treated with chemotherapy
Cancer patients who received chemotherapy showed reduced activation in the left precuneus, a region thought to play a pivotal role in a wide spectrum of highly integrated tasks, including episodic memory retrieval, visuospatial imagery and self-referential processing (Cavanna & Trimble, 2006). This data aligns with results from a previous ALE analysis by Kesler (2014), which revealed decreased activation in the precuneus of CTx+ patients. Findings are also consistent with a number of studies utilising resting-state fMRI (Chen et al., 2019; Simó et al., 2018), where decreased functional connectivity was observed in the left precuneus in CTx+ patients compared to healthy controls. However, our finding suggests that the alteration in the left precuneus may be a chemotherapy-specific effect. This is supported by the fact that our finding was identified in the contrast with cancer patients who had not received chemotherapy as a control group, hence accounting for variables that may arise from a cancer diagnosis (e.g., anxiety and depression) and surgery (e.g., post-operative dysfunction and inflammation; Li & Caeyenberghs, 2018). Cancer patients from the two studies, which reported coordinates of significant reduction in brain activation within the precuneus (de Ruiter et al., 2011; Stouten-Kemperman et al., 2015), were treated with a combination of fluorouracil, epirubicin and cyclophosphamide chemotherapeutic drugs. Although we cannot disentangle the specific cellular and molecular mechanisms underlying this potential brain alteration, these agents have been found in animal models to permeate the blood-brain-barrier and attack neurons in the CNS through mechanisms involving increased oxidative stress, demyelination and mitochondria disruption (for a review see Lv et al., 2020).
The precuneus may be particularly vulnerable to the neurotoxicity of chemotherapy drugs, given its high metabolic demands. The precuneus is a vastly connected hub that is part of a broader subnetwork (i.e., the default mode network; Utevsky et al., 2014). Due to its widespread connections with associated cortical and subcortical regions, the precuneus is considered functionally valuable for integrative processes (Cavanna & Trimble, 2006). Recent studies leveraging functional connectomes have also found that hub regions have higher metabolic demands and longer-distance connections compared to other brain regions, and could therefore be considered biologically costly (Crossley et al., 2014; Gollo et al., 2018). This high metabolic cost has been revealed in a wide array of clinical populations (Klaassens et al., 2017; Raizman et al., 2020), implicating the precuneus as a region that is often vulnerable to disease and ageing processes. Due to its high metabolic demand, it is possible that the precuneus receives more exposure to chemotherapy neurotoxicity, increasing susceptibility to direct damage and/or indirect disruptions to metabolic resources (Kesler & Blayney, 2016; Mounier et al., 2020). Further, chemotherapy mechanisms may exacerbate physiological cascades that are already impacted by ageing and disease, especially given the sample from our significant result were older cancer survivors (age > 56 years). With these considerations in mind, we provide novel evidence of chemotherapy-induced alterations in the left precuneus which may contribute to cognitive deficits in cancer patients.
Interestingly we did not find other brain regions of significant convergence across the 12 included studies. In particular, the lack of significant findings for the increased contrasts (CTx+ > HC; CTx+ > CTx–) was against expectations. At closer inspection, only 25 foci of significant increases in brain activation in CTx+ compared to controls (across 5 individual studies) in our ALE meta-analysis could be identified. The low number of foci can partially explain the non-significant findings of our meta-analysis. Similarly, our ALE meta-analysis did not reveal significant findings for the CTx+ < HC contrast, possibly due to the low number of areas of significant decreases in CTx + patients compared to healthy controls (21 foci, across 8 individual studies). The ALE method combines significant activation coordinates across multiple studies to uncover which brain regions are most frequently implicated in the literature (Eickhoff et al., 2012: Eickhoff et al., 2009). Moreover, the non-significant findings suggest that the brain regions, which revealed differences in activation in the individual studies, stem from widespread brain regions and therefore the meta-analysis cannot detect significant regions of convergence.
In addition, heterogeneity in the samples across the included studies may have hindered convergence in our meta-analysis (Muller et al., 2018). It has been suggested that functional brain alterations may follow a pattern which progresses over time, with increased compensatory brain activation occurring shortly after chemotherapy cessation and normalising over time, and decreased brain activation persisting over time (Koppelmans et al., 2012; Simó et al., 2013; Sousa et al., 2020). For example, the time between completing chemotherapy and fMRI scan varied considerably among studies. Five studies examined the acute effects of chemotherapy (i.e., < six months post-treatment) of which nearly half found increased cortical activation in CTx+ patients (2 studies, 40%; Kesler et al., 2009; Pergolizzi et al., 2019). Our finding of reduced activation in the precuneus in cancer patients who received chemotherapy compared to no chemotherapy controls was sourced from two studies (de Ruiter et al., 2011; Stouten-Kemperman et al., 2015), which tested cancer survivors in the chronic stage (i.e., > 10 years post-treatment). We suggest that increased brain activation in cortical regions could not be observed due to the combined acute and chronic effects of chemotherapy examined. To this end, convergence of activation in the precuneus in the CTx+ < CTx– contrast may have been found due to a number of communalities across the two studies (from the same research institutes) reporting this finding, including the use of similar inclusion/exclusion selection criteria for cancer patients (e.g., the type of chemotherapeutic agent), the fMRI task (i.e., episodic memory) and scanning protocols (de Ruiter et al., 2011; Stouten-Kemperman et al., 2015).
Correlation between functional brain alterations and cognition
A review of the fMRI literature demonstrated that the majority of previous studies (5/7) did not find an association between altered brain activation patterns and cognitive performance in CTx+ patients. Only two of these studies reported a significant relationship and only one study (de Ruiter et al., 2011) provided sufficient information to compute an effect size. Specifically, we found a strong correlation between altered brain activation and cognitive functioning, such that increased pathology predicts poorer performance on cognitive tasks (i.e., executive function and episodic memory) following chemotherapy. Despite the limited evidence, several functional neuroimaging studies have characterised brain function profiles as ‘biomarkers’ of cognitive functioning (e.g., Kesler et al., 2011). We suggest that these interpretations may be premature and future research examining the relationship between brain-based measures and detailed cognitive assessments is warranted.
Limitations and future directions
There are three notable limitations that impact interpretation of the current results. First is the relatively small number of task-related fMRI studies that have been published. The cluster of reduced activation in the precuneus was found from two of four studies in the CTx+ < CTx− contrast (Table 2). Considering Eickhoff et al. (2016) recommends around 17–20 studies in each dataset to obtain robust results, our ALE analyses may not hold sufficient power to detect small effects, partial out subject-specific variation and ensure that results are not led by single experiments (Muller et al., 2018). Nonetheless, our meta-analysis has combined the most fMRI data on this topic to date. To consolidate findings and clarify other neural abnormalities in CTx+ patients, additional task-related fMRI studies need to be conducted to run a comprehensive meta-analysis on the same topic with a larger sample (e.g., 20 studies) in the future.
The second limitation of the present study is the variation in clinical sample characteristics across the selected fMRI studies (as can be seen in Appendix 2). First, the mix of chemotherapeutic drug combinations may have resulted in fewer robust patterns. Animal models have shown that some agents are more neurotoxic than others (Christie et al., 2012; Yan et al., 2015). For example, studies included a combination of blood-brain-barrier permeable (e.g., methotrexate, fluorouracil, cyclophosphamide) and impermeable chemotherapeutic agents (e.g., doxorubicin), which can induce mixed effects on the CNS (Carozzi et al., 2015). Second, as raised above, the variability in post-chemotherapy time intervals may facilitate divergent brain activation patterns. With the studies ranging from over one-month (Pergolizzi et al., 2019) to 13 years (Stouten-Kemperman et al., 2015) after chemotherapy, it is possible that a mixture of transient compensatory mechanisms and long-term reduced brain alterations are present within this literature. The current number of available studies is too small to separate data according to the abovementioned variables. As the field progresses and more studies are published, we can pool data based on these clinical subgroups and consequently, discover further patterns of altered brain activation in CTx+ patients.
The third limitation of our study is that analyses may be hampered by inconsistencies across the methodological features of the included studies (as can be seen in Table 1). First, the fMRI tasks utilised varied across studies; they comprised an assortment of executive function (i.e., Tower of London), memory (i.e., paired associates learning and levels of processing) and attention (i.e., n-back) tasks. This is a relevant issue, as tasks belonging to different neuropsychological domains activate different brain regions (Simó et al., 2013). Future research would benefit from employing fMRI tasks from the Human Connectome Project dataset (Barch et al., 2013), which have been well-validated and demonstrate reliable activation in function-specific regions, producing data amenable to concatenation. Second, the results of ALE meta-analyses are guided by statistically significant regions identified in previous studies in the form of stereotaxic coordinates (Eickhoff et al., 2009; Eickhoff et al., 2012). The probability of the activity of a voxel or cluster differing between groups is largely affected by the choice of statistical threshold applied. In our meta-analysis, 58% of studies employed low uncorrected thresholds. Studies using less stringent statistical thresholds are more likely to report findings that are significant than studies with stricter thresholds (Acar et al., 2018). This undoubtedly explains some of the variability observed in the literature on functional brain alterations in cancer patients. While ALE is able to remove some between-study variability (i.e., sample size), the fact that the analysis exclusively combines data for statistically significant sites only, suggests it may introduce bias (Acar et al., 2018). Future researchers should take this bias into account by applying cluster-level family-wise error correction (p < .05), as this thresholding procedure has low susceptibility to false positives (Muller et al., 2018).
Despite these limitations, patterns of abnormal brain activation are emerging from task-related fMRI findings on cancer survivors. A strength of the current study lies in the ALE approach used to collate data across diverse neuroimaging studies to clarify known inconsistencies (Sousa et al., 2020). We also performed the most up-to-date literature search of task-related fMRI data on cognitive deficits in cancer patients. For example, compared to a previous ALE analysis (Kesler, 2014), we included six additional studies that were not previously incorporated (Correa et al., 2017; Menning et al., 2017; Pergolizzi et al., 2019; Stouten-Kemperman et al., 2015; Vardy et al., 2019; Wang et al., 2016).
Conclusion
The present ALE meta-analysis is one of the first to combine a series of recent task-related fMRI studies to identify reliable regions of altered brain activation patterns in CTx+ patients. After accounting for cancer-related variables, we found a pattern of reduced activation in the left precuneus of CTx+ patients. Our results provide insight into a possible chemotherapy-induced alteration in cancer survivors, potentially guiding targeted treatment strategies. Further studies are needed to perform a larger-scale meta-analysis using harmonised task-related fMRI protocols, evaluating the effects of chemotherapeutic agents and post-chemotherapy time intervals on brain activation patterns. With continued investigation, fMRI may be considered a useful biomarker of cognitive deficits in the future.
Data availability
The data can be made available upon request to the corresponding author.
References
References marked with an asterisk indicate studies included in the meta-analysis.
Acar, F., Seurinck, R., Eickhoff, S. B., & Moerkerke, B. (2018). Assessing robustness against potential publication bias in activation likelihood estimation (ALE) meta-analyses for fMRI. PLoS One, 13(11), 1–23. https://doi.org/10.1371/journal.pone.0208177
Ahles, T. A., Saykin, A. J., Furstenberg, C. T., Cole, B., Mott, L. A., Skalla, K., et al. (2002). Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. Journal of Clinical Oncology, 20(2), 485–493. https://doi.org/10.1200/JCO.2002.20.2.485
Andryszak, P., Wilkość, M., Izdebski, P., & Żurawski, B. (2017). A systematic literature review of neuroimaging studies in women with breast cancer treated with adjuvant chemotherapy. Contemporary Oncology (Poznan, Poland), 21(1), 6–15. https://doi.org/10.5114/wo.2017.66652
Apple, A. C., Schroeder, M. P., Ryals, A. J., Wagner, L. I., Cella, D., Shih, P. A., et al. (2018). Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage: Clinical, 20, 110–118. https://doi.org/10.1016/j.nicl.2018.07.0210
Askren, M. K., Jung, M., Berman, M. G., Zhang, M., Therrien, B., Peltier, S., et al. (2014). Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: A prospective fMRI investigation. Breast Cancer Research and Treatment, 147(2), 445–455. https://doi.org/10.1007/s10549-014-3092-6
Barch, D. M., Burgess, G. C., Harms, M. P., Petersen, S. E., Schlaggar, B. L., Corbetta, M., et al. (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. https://doi.org/10.1016/j.neuroimage.2013.05.033
Boykoff, N., Moieni, M., & Subramanian, S. K. (2009). Confronting chemobrain: An in-depth look at survivors' reports of impact on work, social networks, and health care response. Journal of Cancer Survivorship: Research and Practice, 3(4), 223–232. https://doi.org/10.1007/s11764-009-0098-x
Briones, T. L., & Woods, J. (2011). Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neuroscience, 12, 1–13. https://doi.org/10.1186/1471-2202-12-124
Carozzi, V. A., Canta, A., & Chiorazzi, A. (2015). Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neuroscience Letters, 596, 90–107. https://doi.org/10.1016/j.neulet.2014.10.014
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129, 564–583. https://doi.org/10.1093/brain/awl004
Chen, B. T., Jin, T., Patel, S. K., Ye, N., Ma, H., Wong, C. W., et al. (2019). Intrinsic brain activity changes associated with adjuvant chemotherapy in older women with breast cancer: A pilot longitudinal study. Breast Cancer Research and Treatment, 176(1), 181–189. https://doi.org/10.1007/s10549-019-05230-y
Christie, L. A., Acharya, M. M., Parihar, V. K., Nguyen, A., Martirosian, V., & Limoli, C. L. (2012). Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clinical Cancer Research, 18(7), 1954–1965. https://doi.org/10.1158/1078-0432.CCR-11-2000
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Lawrence Erlbaum.
*Conroy, S. K., McDonald, B. C., Smith, D. J., Moser, L. R., West, J. D., Kamendulis, L. M., … Saykin, A. J. (2013a). Alterations in brain structure and function in breast cancer survivors: Effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Research and Treatment, 137(2), 493–502. https://doi.org/10.1007/s10549-012-2385-x.
Conroy, S. K., McDonald, B. C., Ahles, T. A., West, J. D., & Saykin, A. J. (2013b). Chemotherapy-induced amenorrhea: A prospective study of brain activation changes and neurocognitive correlates. Brain Imaging and Behavior, 7(4), 491–500. https://doi.org/10.1007/s11682-013-9240-5
Cook, M. J., Gardner, A. J., Stanwell, P., Wojtowicz, M., Williams, W. H., & Iverson, G. L. (2020). Task-related functional magnetic resonance imaging activations in patients with acute and subacute mild traumatic brain injury: A coordinate-based meta-analysis. NeuroImage: Clinical, 25, 1–15. https://doi.org/10.1016/j.nicl.2019.102129
*Correa, D. D., Root, J. C., Kryza-Lacombe, M., Mehta, M., Karimi, S., Hensley, M. L., & Relkin, N. (2017). Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: A pilot study. Brain Imaging and Behavior, 11(6), 1652–1663. https://doi.org/10.1007/s11682-016-9608-4.
Crossley, N. A., Mechelli, A., Scott, J., Carletti, F., Fox, P. T., McGuire, P., & Bullmore, E. T. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. BRAIN, 137, 2382–2395. https://doi.org/10.1093/brain/awu132
de Ruiter, M. B., & Schagen, S. B. (2013). Functional MRI studies in non-CNS cancers. Brain Imaging and Behavior, 7(4), 388–408. https://doi.org/10.1007/s11682-013-9249-9
*de Ruiter, M. B., Reneman, L., Boogerd, W., Veltman, D. J., van Dam, F. S., Nederveen, A. J., … Schagen, S. B. (2011). Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human Brain Mapping, 32(8), 1206–1219. https://doi.org/10.1002/hbm.21102.
Deprez, S., Vandenbulcke, M., Peeters, R., Emsell, L., Smeets, A., Christiaens, M. R., Amant, F., & Sunaert, S. (2014). Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. Journal of Clinical Oncology, 32(19), 2031–2038. https://doi.org/10.1200/JCO.2013.53.6219
Eickhoff, S. B., Grefkes, C., Wang, L. E., Zilles, K., Laird, A. R., & Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. https://doi.org/10.1002/hbm.20718
Eickhoff, S. B., Bzdok, D., Laird, A. R., Fox, P. T., & Kurth, F. (2012). Activation likelihood estimation meta-analysis revisited. NeuroImage, 59(3), 2349–2361. https://doi.org/10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Nichols, T. E., Laird, A. R., Hoffstaedter, F., Amunts, K., Fox, P. T., et al. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. https://doi.org/10.1016/j.neuroimage.2016.04.072
Ferguson, R. J., McDonald, B. C., Saykin, A. J., & Ahles, T. A. (2007). Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. Journal of Clinical Oncology, 25(25), 3866–3870. https://doi.org/10.1200/JCO.2007.10.8639
Fuelscher, I., Caeyenberghs, K., Enticott, P. G., Williams, J., Lum, J., & Hyde, C. (2018). Differential activation of brain areas in children with developmental coordination disorder during tasks of manual dexterity: An ALE meta-analysis. Neuroscience and Biobehavioral Reviews, 86, 77–84. https://doi.org/10.1016/j.neubiorev.2018.01.002
Gollo, L. L., Roberts, J. A., Cropley, V. L., Di Biase, M. A., Pantelis, C., Zalesky, A., & Breakspear, M. (2018). Fragility and volatility of structural hubs in the human connectome. Nature Neuroscience, 21(8), 1107. https://doi.org/10.1038/s41593-018-0188-z
Henderson, F. M. E., Cross, A. J., & Baraniak, A. R. (2019). ‘A new normal with chemobrain’: Experiences of the impact of chemotherapy-related cognitive deficits in long-term breast cancer survivors. Health Psychology Open, 6(1). https://doi.org/10.1177/2055102919832234
Hosseini, S. M. H., & Kesler, S. R. (2014). Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. Journal of the International Neuropsychological Society : JINS, 20(4), 391–401. https://doi.org/10.1017/S1355617713001173
Hutchinson, A. D., Hosking, J. R., Kichenadasse, G., Mattiske, J. K., & Wilson, C. (2012). Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews, 38(7), 926–934. https://doi.org/10.1016/j.ctrv.2012.05.002
Janelsins, M. C., Kesler, S. R., Ahles, T. A., & Morrow, G. R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry, 26(1), 102–113. https://doi.org/10.3109/09540261.2013.864260
Jansen, C. E., Cooper, B. A., Dodd, M. J., & Miaskowski, C. A. (2011). A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Supportive Care in Cancer, 19(10), 1647–1656. https://doi.org/10.1007/s00520-010-0997-4
Jung, M. S., Zhang, M., Askren, M. K., Berman, M. G., Peltier, S., Hayes, D. F., Therrien, B., Reuter-Lorenz, P. A., & Cimprich, B. (2017). Cognitive dysfunction and symptom burden in women treated for breast cancer: A prospective behavioral and fMRI analysis. Brain Imaging and Behavior, 11(1), 86–97. https://doi.org/10.1007/s11682-016-9507-8
Kam, J. W. Y., Boyd, L. A., Hsu, C. L., Liu-Ambrose, T., Handy, T. C., Lim, H. J., Hayden, S., & Campbell, K. L. (2016). Altered neural activation during prepotent response inhibition in breast cancer survivors treated with chemotherapy: An fMRI study. Brain Imaging and Behavior, 10(3), 840–848. https://doi.org/10.1007/s11682-015-9464-7
Kesler, S. R. (2014). Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiology of Aging, 35(2), 11–19. https://doi.org/10.1016/j.neurobiolaging.2014.03.036
Kesler, S. R., & Blayney, D. W. (2016). Neurotoxic effects of anthracycline vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncology, 2(2), 185–192. https://doi.org/10.1093/jnci/djv131
*Kesler, S. R., Bennett, F. C., Mahaffey, M. L., & Spiegel, D. (2009). Regional brain activation during verbal declarative memory in metastatic breast cancer. Clinical Cancer Research, 15(21), 6665–6673. https://doi.org/10.1158/1078-0432.CCR-09-1227.
*Kesler, S. R., Kent, J. S., & O'Hara, R. (2011). Prefrontal cortex and executive function impairments in primary breast cancer. Archives of Neurology, 68(11), 1447–1453. https://doi.org/10.1001/archneurol.2011.245.
Klaassens, B. L., van Gerven, J. M. A., van der Grond, J., de Vos, F., Möller, C., & Rombouts, S. A. R. B. (2017). Diminished posterior precuneus connectivity with the default mode network differentiates normal aging from Alzheimer’s disease. Frontiers in Aging Neuroscience, 9, 1–13. https://doi.org/10.3389/fnagi.2017.00097
Koppelmans, V., Breteler, M. M. B., Boogerd, W., Seynaeve, C., Gundy, C., & Schagen, S. B. (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology, 30(10), 1080–1086. https://doi.org/10.1200/JCO.2011.37.0189
Lacadie, C. M., Fulbright, R. K., Rajeevan, N., Constable, R. T., & Papademetris, X. (2008). More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage, 42(2), 717–725. https://doi.org/10.1016/j.neuroimage.2008.04.240
Li, M., & Caeyenberghs, K. (2018). Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: A systematic review. Neuroscience and Biobehavioral Reviews, 92, 304–317. https://doi.org/10.1016/j.neubiorev.2018.05.019
*López Zunini, R. A., Scherling, C., Wallis, N., Collins, B., MacKenzie, J., Bielajew, C., & Smith, A. M. (2013). Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: A prospective fMRI study. Brain Imaging and Behavior, 7(4), 460–477. https://doi.org/10.1007/s11682-012-9213-0.
Lv, L., Mao, S., Dong, H., Hu, P., & Dong, R. (2020). Pathogenesis, assessments and management of chemotherapy-related cognitive impairment (CRCI): An updated literature review. Journal of Oncology, 2020. https://doi.org/10.1155/2020/3942439
*McDonald, B. C., Conroy, S. K., Ahles, T. A., West, J. D., & Saykin, A. J. (2012). Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. Journal of Clinical Oncology, 30(20), 2500–2508. https://doi.org/10.1200/JCO.2011.38.5674.
*Menning, S., De Ruiter, M. B., Veltman, D. J., Boogerd, W., Oldenburg, H. S. A., Reneman, L., & Schagen, S. B. (2017). Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLoS One, 12(3), 1–16. https://doi.org/10.1371/journal.pone.0171724
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), 1–97. https://doi.org/10.1371/journal.pmed.1000097
Mounier, N. M., Abdel-Maged, A. E.-S., Wahdan, S. A., Gad, A. M., & Azab, S. S. (2020). Chemotherapy-induced cognitive impairment (CICI): An overview of etiology and pathogenesis. Life Sciences, 258, 1–14. https://doi.org/10.1016/j.lfs.2020.118071
Muller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 84, 151–161. https://doi.org/10.1016/j.neubiorev.2017.11.012
Myers, J. S. (2012). Chemotherapy-related cognitive impairment: The breast cancer experience. Oncology Nursing Forum, 39(1), 31–40. https://doi.org/10.1188/12.ONF.E31-E40
*Pergolizzi, D., Root, J. C., Pan, H., Silbersweig, D., Stern, E., Passik, S. D., & Ahles, T. A. (2019). Episodic memory for visual scenes suggests compensatory brain activity in breast cancer patients: A prospective longitudinal fMRI study. Brain Imaging and Behavior, 13(6), 1674–1688. https://doi.org/10.1007/s11682-019-00038-2.
Pomykala, K. L., de Ruiter, M. B., Deprez, S., McDonald, B. C., & Silverman, D. H. S. (2013). Integrating imaging findings in evaluating the post-chemotherapy brain. Brain Imaging & Behavior, 7(4), 436–452. https://doi.org/10.1007/s11682-013-9239-y
Raizman, R., Tavor, I., Biegon, A., Harnof, S., Hoffmann, C., Tsarfaty, G., et al. (2020). Traumatic brain injury severity in a network perspective: A diffusion MRI based connectome study. Scientific Reports, 10(1), 1–12. https://doi.org/10.1038/s41598-020-65948-4
Santangelo, G., Raimo, S., Cropano, M., Vitale, C., Barone, P., & Trojano, L. (2019). Neural bases of impulse control disorders in Parkinson's disease: A systematic review and an ALE meta-analysis. Neuroscience and Biobehavioral Reviews, 107, 672–685. https://doi.org/10.1016/j.neubiorev.2019.09.041
Scherling, C. S., & Smith, A. (2013). Opening up the window into "chemobrain": A neuroimaging review. Sensors, 13(3), 3169–3203. https://doi.org/10.3390/s130303169
Simó, M., Rifà-Ros, X., Rodriguez-Fornells, A., & Bruna, J. (2013). Chemobrain: A systematic review of structural and functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(8), 1311–1321. https://doi.org/10.1016/j.neubiorev.2013.04.015
Simó, M., Rifà-Ros, X., Vaquero, L., Ripollés, P., Cayuela, N., Jové, J., et al. (2018). Brain functional connectivity in lung cancer population: An exploratory study. Brain Imaging & Behavior, 12(2), 369–382. https://doi.org/10.1007/s11682-017-9697-8
Sousa, H., Almeida, S., Bessa, J., & Pereira, M. G. (2020). The developmental trajectory of cancer-related cognitive impairment in breast cancer patients: A systematic review of longitudinal neuroimaging studies. Neuropsychology Review, 30(3), 287–309. https://doi.org/10.1007/s11065-020-09441-9
*Stouten-Kemperman, M. M., de Ruiter, M. B., Boogerd, W., Veltman, D. J., Reneman, L., & Schagen, S. B. (2015). Very late treatment-related alterations in brain function of breast cancer survivors. Journal of the International Neuropsychological Society, 21(1), 50–61. https://doi.org/10.1017/S1355617714001015.
Stouten-Kemperman, M. M., De Ruiter, M. B., Boogerd, W., Kerst, J. M., Kirschbaum, C., Reneman, L., & Schagen, S. B. (2018). Brain Hyperconnectivity >10 years after cisplatin-based chemotherapy for testicular Cancer. Brain Connectivity, 8(7), 398–406. https://doi.org/10.1089/brain.2017.0569
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians., 71(3), 209–249. https://doi.org/10.3322/caac.21660
Turkeltaub, P. E., Wiener, M., Eickhoff, S. B., Laird, A. R., Fox, M., & Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13. https://doi.org/10.1002/hbm.21186
Utevsky, A. V., Smith, D. V., & Huettel, S. A. (2014). Precuneus is a functional core of the default-mode network. Journal of Neuroscience, 34, 932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
*Vardy, J. L., Stouten-Kemperman, M. M., Pond, G., Booth, C. M., Rourke, S. B., Dhillon, H. M., … Tannock, I. F. (2019). A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging and Behavior, 13(1), 15–26. https://doi.org/10.1007/s11682-017-9728-5.
*Wang, L., Apple, A. C., Schroeder, M. P., Ryals, A. J., Voss, J. L., Gitelman, D., … Wagner, L. I. (2016). Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors post-chemotherapy compared with healthy controls. Cancer, 122(2), 258–268. https://doi.org/10.1002/cncr.29737.
Wefel, J. S., Saleeba, A. K., Meyers, C. A., & Buzdar, A. U. (2010). Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer, 116(14), 3348–3356. https://doi.org/10.1002/cncr.25098
Yan, F., Liu, J. J., Ip, V., Jamieson, S. M. F., & McKeage, M. J. (2015). Role of platinum DNA damage-induced transcriptional inhibition in chemotherapy-induced neuronal atrophy and peripheral neurotoxicity. Journal of Neurochemistry, 135(6), 1099–1112. https://doi.org/10.1111/jnc.13355
Zhang, B., Lin, P., Shi, H., Öngür, D., Auerbach, R., Wang, X., et al. (2016). Mapping anhedonia-specific dysfunction in a transdiagnostic approach: An ALE meta-analysis. Brain Imaging & Behavior, 10(3), 920. https://doi.org/10.1007/s11682-015-9457-6
Code availability
Code used via Ginger ALE version 2.3.6 is available through http://brainmap.org.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (JBS and KC), data collection or acquisition (JBS and EGE), statistical analysis (JBS, EGE and KC), interpretation of results (JBS, EGE, ALC and KC), drafting the manuscript work or revising it critically for important intellectual content (JBS, EGE, ALC and KC) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (JBS, EGE, ALC and KC).
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
None of the authors have a conflict of interest to declare.
Ethics approval
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1 Full Search Syntax Used in Systematic Review
Appendix 2 Clinical Population Characteristics
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saward, J.B., Ellis, E.G., Cobden, A.L. et al. Mapping cognitive deficits in cancer patients after chemotherapy: An Activation Likelihood Estimation meta-analysis of task-related fMRI studies. Brain Imaging and Behavior 16, 2320–2334 (2022). https://doi.org/10.1007/s11682-022-00655-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00655-4