Abstract
The relation of white matter hyperintense lesions to episodic memory impairment in patients with Parkinson’s disease (PD) is still controversial. We aimed at evaluating the relation between white matter hyperintense lesions and episodic memory decline in patients with PD. In this multicentric prospective study, twenty-one normal controls, 15 PD patients without mild cognitive impairment (MCI) and 13 PD patients with MCI were selected to conduct a clinico-radiological correlation analysis. Performance during episodic memory testing, age-related white matter changes score, total manual and automated white matter hyperintense lesions volume and lobar white matter hyperintense lesions volumes were compared between groups using the Kruskal-Wallis and Wilcoxon signed-rank tests, and correlations were assessed using the Spearman test. MCI PD patients had impaired free recall. They also had higher total, left prefrontal and left temporal white matter hyperintense lesions volumes than normal controls. Free recall performance was negatively correlated with the total white matter hyperintense lesions volume, either manually or automatically delineated, but not with the age-related white matter changes score. Using automated segmentation, both the left prefrontal and temporal white matter hyperintense lesions volumes were negatively correlated with the free recall performance. Early episodic memory impairment in MCI PD patients may be related to white matter hyperintense lesions, mainly in the prefrontal and temporal lobes. This relation is influenced by the method used for white matter hyperintense lesions quantification. Automated volumetry allows for detecting those changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive dysfunction in elderly subjects may be linked to white matter hyperintense lesions (WMHL) related to cerebral small vessel disease (CSVD) (Zhou et al. 2015; Maillard et al. 2012; Smith et al. 2011). In elderly hypertensive patients with subjective memory complaints, higher WMHL volume has been correlated with lower grey matter metabolism, independently of age, gender or grey matter atrophy (Verger et al. 2016). Higher WMHL load has also been reported in mild cognitive impaired (MCI) and demented patients with Parkinson’s disease (PD) (Beyer et al. 2006; Kandiah et al. 2013; Lee et al. 2010; Mak et al. 2015; Slawek et al. 2013; Sunwoo et al. 2014). Episodic memory (EM) is early impaired in PD and may be a risk factor for future cognitive decline (Broeders et al. 2013). The impact of CSVD on EM is however still inconclusive, notably in old-age onset PD patients. A recent critical review of the pertinent literature (Vesely and Rektor 2016) suggested that the controversial results on the contribution of WMHL to cognitive decline in PD might be due to differences in methods of cognitive testing and WMHL load assessment from MRI.
WMHL load can be evaluated either by visual rating or quantitative methods. While visual rating and WMHL volume have been well correlated (Kapeller et al. 2003) and present good interrater reliability (Olsson et al. 2013), an automatized volumetric method for measuring WMHL load is more suitable for evaluating progression of the disease (Prins et al. 2004). WMHL volume on fluid-attenuated inversion recovery (FLAIR) images may be delineated manually, using semi-automated (Dalaker et al. 2009) or fully-automated software (Fartaria et al. 2016; Gibson et al. 2010), this latter demonstrating high accuracy. We thus hypothesized in the present study that early EM decline in elderly MCI patients with newly diagnosed PD could be due to CSVD and that this relation could be influenced by the employed WMHL quantification method.
The aim of the study was to evaluate the relation between WMHL load and EM testing in patients older than 70 years with newly diagnosed PD as well as the impact of the WMHL evaluation method on this interrelation.
Materials and Methods
Study population
From December 2011 to June 2016, 27 healthy controls (mean age: 77.6 ± 3.2 years; male: 20) and 67 patients (mean age: 79.6 ± 4.7 years; male: 35) were prospectively enrolled in this multicentric study. Inclusion criteria for patients were: age between 70 and 90 years, symptoms of parkinsonism starting after the age of 70 years, no history of cardiovascular disease. Inclusion criteria for controls were: age between 70 and 90 years, no parkinsonian symptoms, no history of cardiovascular disease. Exclusion criteria were identical for all subjects (controls and patients) and included: previous history of head injury, stroke, neuroinflammatory disorders, myocardial infarct, intra-cranial bleeding, exposure to neuroleptic drugs, psychiatric comorbidity, contraindications to MRI. In order to rule out stroke, intra-cranial bleeding or neuroinflammatory disorders related sequelae, brain MRI or CT examinations available on the institutional image archiving system before inclusion as well as MRI scans performed during the study were reviewed by a neuroradiologist who was not involved in the MRI data processing. All participants underwent brain MRI, a neurological examination to determine the parkinsonism’s subtype and a neuropsychological assessment including EM testing within the same day. The mean delay between patients’ inclusion and clinico-radiological evaluation was 9.4 ± 42.4 days.
Ethical approval
This study was approved by the Institutional Review Board and conformed with the World Medical Association Declaration of Helsinki. The experiments were undertaken with understanding and written informed consent of each subject.
Clinical assessment
All participants underwent a neurological and neuropsychological evaluation on the day of brain MRI. Diagnoses were established by a neurologist experienced in parkinsonian syndromes, blinded to the MRI results and according to the following established guidelines: the UK Parkinson’s Disease Society Brain Bank criteria for idiopathic PD, the National Institute of Neurological Disorders and Stroke and the Society for Progressive Supranuclear Palsy (PSP) criteria for PSP, Zijlmans’s criteria along with multiple cardiovascular risk factors and minimal or absent Levodopa response for vascular parkinsonism (VP), the Gilman’s criteria for multiple system atrophy (MSA), and the Lang criteria for corticobasal dementia (CBD) as previously reported (Dunet et al. 2016).
Neuropsychological assessment included testing of five cognitive domains: attention and working memory, executive functions, language, visuospatial abilities and episodic memory. Global cognitive efficiency was assessed using the Mini Mental State Examination (MMSE) score (range 0–30) and the Mattis Dementia Rating Scale (range 0–144), which involves five subsets (attention, initiation, construction, conceptualization, and memory). Attention and working memory were assessed with a auditory-verbal forward and backward span task and the Delis-Kaplan Executive Function System (D-KEFS) Trail Making Test evaluating flexibility; executive functions were assessed with the Frontal Assessment Battery, the french version of the Stroop Victoria test evaluating inhibition, the D-KEFS Trail Making Test evaluating flexibility, the Rey complex figure copy test evaluating planification abilities and the clock-drawing test; language was evaluated with the LEXIS and Isaac’s Set tests; visuospatial functions were assessed with the Rey complex figure copy test. Finally, to assess EM, we used the free/cued recall selective reminding test (FCSRT) (Grober et al. 2010). After a training phase that controls verbal attention and semantic encoding, the study includes three trials of recall for 16 items, separated by 20 s of interference, to explore retrieval. Each trial of recall consists of free recall followed by cued recall. A single delayed free/cued recall trial is finally performed 20 min later. Neuropsychological metrics were recorded as z-scores (free recall) or percentiles (cued recall) compared with results of a healthy population normalized for age and gender. MCI patients were diagnosed according to the Level I of the Movement Disorders Task Force guidelines (Litvan et al. 2012) when at least two neuropsychological tests in the five domains were impaired. The Educational Attainment was also recorded.
Images acquisition and processing
All subjects underwent brain MRI on a 3 Tesla scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) including an axial unenhanced T1-weighted magnetization-prepared rapid 3D gradient-echo (MPRAGE) sequence (repetition time = 1690 ms, echo time = 2.54 ms, inversion time = 922 ms, turbo actor = 208, flip angle = 9°, slice thickness = 1 mm, 176 slices, isotropic voxel size = 1 mm3) and sagittal FLAIR sequence (repetition time = 5000 ms, echo time = 384 ms, inversion time = 1800 ms, flip angle = 120°, slice thickness = 0.9 mm, 160 slices, interpolated voxel size = 0.9 × 0.9 × 0.9 mm3).
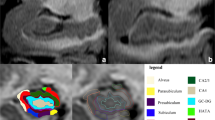
WMHL load was evaluated by visual rating as well as by both manual and automated volume estimation at the patient level. For visual rating, FLAIR images were reviewed by two neuroradiologists in consensus to measure the severity of cerebral age-related white matter changes (ARWMC) by using the four-point scale of the European Task Force: 0 (no lesion), 1 (focal non confluent lesions ≥5 mm), 2 (beginning confluence of lesions) or 3 (diffuse involvement). For WMHL volume estimation, WMHL were manually contoured on FLAIR images by a single neuroradiologist with 8-years’ experience in neuroimaging with the MRIcron software (https://www.nitrc.org/projects/mricron). Volumes were recorded in milliliters (mL) and as percentage of the total intracranial volume. To ensure reproducibility of the measure, delineation was repeated twice for ten subjects randomly chosen, while respecting a delay of 3 months between the two delineations. Mean delineation time was about 20 min per case (1880 min for all participants).
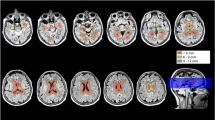
Finally, WMHL volume was also computed using automated prototype software initially designed for Multiple Sclerosis lesion segmentation (Fartaria et al. 2016). The method consists of two main steps: i) pre-processing, where the images are aligned, skull-striped, corrected for bias field and intensity-normalized; and ii) lesion segmentation, performed by a supervised classifier based on k-nearest-neighbor (k-NN) algorithm. Lesion masks of each subject were obtained through a “leave-one-out” cross-validation on the whole cohort of 94 subjects (see section Study population). Lobar WMHL volumes were estimated by summing up voxels labelled as lesion tissue by the automated algorithm over the left and right pre-frontal, frontal, temporal, parietal and occipital masks obtained by atlas propagation (Schmitter et al. 2015). The computation time for the automated segmentation was 10 min per patient (940 min for all participants) on a standard computer. T1-MPRAGE images were also segmented to estimate the lobar (pre-frontal, frontal, temporal, parietal, occipital) grey matter and hippocampus volumes using the MorphoBox software (Schmitter et al. 2015).
Statistical analysis
All statistics were performed with the Stata 13.1 software (Stata Corp., College Station, TX, USA). Continuous variables are presented as mean ± standard deviation. Statistical analysis was performed in a two-step manner. First, the correlation and concordance of the three methods to quantify WMHL for all participants (n = 94) was evaluated by the Lin’s test and Bland-Altman plot with computation of 95% limits-of-agreement (LOA). Second, a clinico-radiological correlation analysis was performed to identify if there was a significant relation between high WMHL load and clinical assessment, i.e. EM testing. For this analysis, three groups were considered: normal controls (NC), PD patients without MCI (non-MCI PD), and PD patients with MCI (MCI PD). Group comparisons were performed using the Kruskal-Wallis and Wilcoxon tests for continuous variables and the Fisher exact test for proportions. The relation between each neuropsychological metric (FCSRT and Mattis score) and total WMHL load (ARWMC score, total manual and automated WMHL volumes) was assessed by the non-parametric Spearman rho correlation coefficient. We also evaluated the relation between the same neuropsychological metrics and lobar (left/right prefrontal, frontal, temporal, parietal, occipital) WMHL and grey matter (lobar and hippocampus) volumes by the non-parametric Spearman correlation coefficient. All correlation analyses were adjusted for age and gender. An uncorrected p-value <0.05 was considered significant. For multiple correlation analyses, correction of the significance level was performed using the Benjamini and Hochberg method with a false discovery rate of 0.1 (Green and Diggle 2007). For these multiple correlation analyses, only results remaining significant after correction of the significance level are reported in the text.
Results
Study population
Out of 67 patients who underwent MRI, thirty-four patients met the criteria of idiopathic PD (mean age 79.9 ± 5.2yo; male: 20), eight patients those of VP (mean age 78.0 ± 2.0yo; male: 7), seven patients those of PSP (mean age 79.6 ± 6.7yo; male: 5), four patients those of MSA (mean age: 79.5 ± 3.8yo; male: 2), three patients those of CBD (mean age: 77.3 ± 4.0yo; male: 0), one met criteria for Lewy body dementia (age: 76yo; male: 1), and ten could not yet be classified (mean age: 80.9 ± 4.0yo; male: 4).
Characteristics of subjects included in the clinico-radiological correlation analysis are summarized in Table 1. Out of the 27 controls, six with MCI were excluded. Out of the 34 PD patients, six with dementia were excluded. Overall, 21 NC, 15 non-MCI PD patients and 13 MCI PD patients were included. MCI PD patients were older than NC (p = 0.0001). There was no statistically significant difference regarding sex ratio, educational level, time-from-onset of PD symptoms or dopaminergic therapy. MCI PD patients had impaired free and cued recall, global memory, initiation and conceptualization compared with NC (Table 2). They did not have impaired verbal attention and semantic encoding. Regarding grey matter, only occipital volume was lower in MCI PD patients than in NC (p = 0.014) and non-MCI PD patients (p = 0.019).
WMHL load estimation
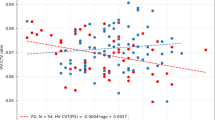
On visual rating, eight subjects had score 0, 34 had score 1, 31 had score 2, and 21 had score 3. Mean total WMHL volumes were 12.7 ± 13.5 mL and 12.5 ± 10.8 mL for manual and automated segmentation, respectively. The concordance between manually and automatically delineated WMHL volumes was excellent (Pearson’s r = 0.974, rho_c = 0.950, C_b = 0.975, mean difference = 0.18 ± 3.9 mL, 95%LOA = −7.43–7.79, p = 0.65). Manual delineation was highly reproducible (Pearson’s rho = 0.997, rho_c = 0.997, C_b = 0.999, mean difference = 0.07 ± 1.0 mL, 95%LOA = −1.9–2.1, p = 0.83). Both were positively correlated with ARWMC score (rho = 0.90, p < 0.0001 and rho = 0.87, p < 0.0001 for manual and automated segmentation, respectively, Fig. 1).
ARWMC score was not different between groups while total WMHL volumes tend to be higher in MCI PD patients than in NC (p = 0.058 and p = 0.063 for manual and automated segmentation, respectively). Total WMHL volume was not correlated with age in our cohort (rho =0.11, p = 0.45). Lobar WMHL volumes are displayed in Table 1.
Clinico-radiological correlation
Detailed results of the correlation analysis between total WMHL volume and neuropsychological metrics are displayed in Table 3. ARWMC score was not correlated with any neuropsychological metric. Total WMHL volume estimated by manual segmentation was correlated with performance during the second cued recall.
Using automated segmentation, performances during the second free recall were correlated with the left prefrontal (rho = −0.37, p = 0.009) and left temporal (rho = −0.35, p = 0.016) WMHL volumes. Performances during the third free recall were correlated with the left prefrontal (rho = −0.35, p = 0.014) and left temporal (rho = −0.39, p = 0.0058) WMHL volumes. Delayed free recall performance was correlated with the left prefrontal (rho = −0.37, p = 0.013) and left temporal (rho = −0.43, p = 0.0035) WMHL volumes. Finally, the left prefrontal WMHL volume was correlated with the Mattis memory subscore (rho = −0.36, p = 0.013). Lobar grey matter and hippocampus volumes were not correlated with any neuropsychological metric (all corrected p > 0.15).
Discussion
In this prospective multicentric study focusing on subjects older than 70 years, PD patients with MCI presented early EM impairment, which was not present in normal controls. EM decline correlated with WMHL volumes, especially in the left prefrontal and temporal lobes, which were accurately quantified by the automated lesion segmentation.
Relation between WMHL and cognitive decline
WMHL related to CSVD have been correlated with cognitive dysfunction in elderly patients with (Malek et al. 2016) or without PD (Zhou et al. 2015; Maillard et al. 2012; Smith et al. 2011). In a large stroke-free population, WMHL was especially associated with worse memory in subjects older than 70 years, independently of brain atrophy (Dong et al. 2015). Similarly, we found that PD patients older than 70 years with MCI presented early EM decline compared with NC, particularly during free recall testing. MCI PD patients were older than NC but all neuropsychological metrics were normalized for age and gender. Thus, age-related confounds do not account for any of the observed differences in EM performance or for the relation between EM and WMHL. Indeed, total WMHL volume was negatively correlated with EM performance. Our findings thus confirm that WMHL due to CSVD may result in worse EM also in subjects older than 70 years with PD. This is in agreement with two other studies (Kandiah et al. 2013; Lee et al. 2010), which demonstrated that high WMHL volume is associated with low memory performance in PD patients, regardless of age, gender, education status, cardiovascular risk factors, disease duration, or dopaminergic therapy – as in our study. Additionally, we found that EM decline was correlated with WMHL volumes in the left prefrontal and temporal lobes, but not with lobar grey matter or hippocampus volumes, which has never been reported in PD patients. Higher WMHL volumes in the prefrontal lobe may result in lower activity in the prefrontal, temporal and cingulate cortex during EM tasks as demonstrated by Nordahl and co-workers in healthy individuals (Nordahl et al. 2006). As in NC (Lancaster et al. 2016) and in MCI non-PD patients (Remy et al. 2015), temporal white matter microstructure disruption due to regional WMHL may also account for early EM impairment in MCI PD patients. Spatial distribution of WMHL could thus sustain EM decline in MCI PD patients. Moreover, we found that PD patients did not have impaired verbal attention and semantic encoding and that attention was not significantly correlated with total or lobar WMHL volumes. This is in agreement with Dalaker et al. (2009) who found no significant relation between total volume or spatial distribution of WMHL and attention-executive function in PD. Notably, the same authors did not explore the relation between WMHL and EM testing. In three other studies, there was no significant relation between cognitive performance and WMHL severity (Slawek et al. 2013, 2008) or between the progression of WMHL and progression to higher category of cognitive impairment (Gonzalez-Redondo et al. 2012). It is worth mentioning that these studies included PD patients with dementia and/or long disease duration, thus potentially mixing CSVD and PD-related neurodegeneration effects. Indeed, Jones and co-workers (2017) recently highlighted that PD and cardiovascular risk factors are independent risk factors for cognitive impairment. Sunwoo et al. (2014) also found that total WMHL volume is an independent predictor of conversion from MCI to dementia in PD patients. Overall, this suggests that, at the early stage of PD, CSVD rather than PD-related neurodegeneration could induce EM decline. At a later stage, CSVD could still contribute to early conversion to dementia along with PD-related neurodegeneration.
Impact of WMHL evaluation method
In a recent critical literature review, Vesely and Rektor (2016) suggested that controversial results on the contribution of WMHL to PD cognitive decline might be due to methodological differences for assessing WMHL on MR images. Indeed, in PD patients, WMHL severity has alternatively been evaluated using qualitative (Rodriguez-Oroz et al. 2009; Ng et al. 2012) or semi-quantitative (Beyer et al. 2006; Gonzalez-Redondo et al. 2012; Lee et al. 2010; Slawek et al. 2008) visual rating as well as semi-automated (Dalaker et al. 2009; Mak et al. 2015) or automated (Sunwoo et al. 2014; Kandiah et al. 2013) volumetric estimation. Compared with NC, we did not find higher WMHL load in MCI PD patients using ARWMC visual rating as several previously published studies (Gonzalez-Redondo et al. 2012; Rodriguez-Oroz et al. 2009; Slawek et al. 2008). Using manual or automated segmentation, total WMHL volume tended to be higher in MCI PD patients than in NC, in agreement with the results of Dalaker and co-workers (2009). Two studies (Kandiah et al. 2013; Mak et al. 2015) additionally found higher total WMHL volume in MCI PD patients compared with non-MCI PD patients. Although manual segmentation was highly reproducible, the performance of automated segmentation was excellent with a notable gain in processing time. This provides evidence that automated WMHL segmentation has the potential to accurately measure WMHL volume in PD patients, as it is the case in patients with multiple sclerosis (Fartaria et al. 2016; Gibson et al. 2010). As suggested by Vesely and Rektor (2016), the type of WMHL estimation method influenced the clinico-radiological correlation analysis in our study. On the one hand, we did not find any significant relation between ARWMC score and neuropsychological metrics, as most studies that used simple visual rating (Gonzalez-Redondo et al. 2012; Rodriguez-Oroz et al. 2009). On the other hand, we found a significant relation between EM metrics and total WMHL volume using both manual and automated segmentation. This is in agreement with three studies, including one that used semi-quantitative visual rating (Lee et al. 2010), one that used semi-automated volume quantification (Mak et al. 2015) and one that used automated volume quantification (Kandiah et al. 2013). Interestingly, we first report a correlation between EM decline of PD patients and WMHL volumes in the left prefrontal and temporal lobes, which can only be reliably obtained by an automated segmentation. This overall suggests that automated WMHL volume quantification is fast, reproducible and more suitable to evaluate the relation between EM impairment and CSVD severity.
Study limitations
This study has several limitations. The sample size in the clinico-radiological correlation analysis was small (n = 49) due to the very stringent inclusion and exclusion criteria for the elderly patients’ population. This, however, avoided confounding factors such as stroke, haemorrhage, or traumatic cerebral disease. Our results were moreover independent of age, gender, educational attainment, cardiovascular risk factors, disease duration, and dopamine therapy. Nevertheless larger studies are needed to confirm our results. Regarding the diagnosis of VP, recent recommendations propose three subtypes: post-stroke VP, insidious VP and mixed PD/CVD (Rektor et al. 2018). While post-stroke VP patients were formally excluded from our study we used modified Zijlman’s criteria to diagnose VP patients, which fit criteria for the insidious VP subtype. In the present study, PD patients and mixed PD/CVD patients were enrolled in the same group due to the absence of clear validation of these new criteria and because nuclear medicine imaging was not available in this cohort. As we based our correlation analyses on a WMHL location-free hypothesis, our results are still valuable. Whether automated WMHL volume could help distinguishing between mixed PD/CVD patients and PD patients without CVD should be investigated. Longitudinal data were not recorded and are needed to evaluate the impact of WMHL volume on the time course of EM decline. As discussed above, it was demonstrated that WMHL is an independent predictor of conversion from MCI to dementia in PD patients (Sunwoo et al. 2014). Although Maillard et al. (2012) demonstrated that a 1 mL/year increase in global WMHL volume is associated with an additional 0.7 SD/year of subsequent EM decrease in non-PD subjects, it remains unclear whether WMHL volume follow-up could predict the individual time course of EM decline in PD patients. While effect of cognitive training on memory remained uncertain in a recent meta-analysis (Leung et al. 2015), it is also unknown whether automated WMHL volumetry could be used as a marker to select and follow-up patients who could benefit from cognitive rehabilitation, aggressive cardiovascular risk factors control, or other alternative therapy. This needs further investigations.
Conclusion
Overall, our results indicate that CSVD may contribute to early EM impairment in MCI PD patients older than 70 years. The relation between neuropsychological metrics and CSVD severity is influenced by the method of assessing WMHL on MRI images. Automated quantification of WMHL volume is reliable and may have diagnostic, prognostic and therapeutic implications in PD patients.
References
Beyer, M. K., Aarsland, D., Greve, O. J., & Larsen, J. P. (2006). Visual rating of white matter hyperintensities in Parkinson's disease. Movement Disorders, 21(2), 223–229. https://doi.org/10.1002/mds.20704.
Broeders, M., Velseboer, D. C., de Bie, R., Speelman, J. D., Muslimovic, D., Post, B., et al. (2013). Cognitive change in newly-diagnosed patients with Parkinson's disease: a 5-year follow-up study. Journal of the International Neuropsychological Society, 19(6), 695–708. https://doi.org/10.1017/S1355617713000295.
Dalaker, T. O., Larsen, J. P., Dwyer, M. G., Aarsland, D., Beyer, M. K., Alves, G., et al. (2009). White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. Neuroimage, 47(4), 2083–2089. https://doi.org/10.1016/j.neuroimage.2009.06.020.
Dong, C., Nabizadeh, N., Caunca, M., Cheung, Y. K., Rundek, T., Elkind, M. S., et al. (2015). Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology, 85(5), 441–449. https://doi.org/10.1212/WNL.0000000000001716.
Dunet, V., Deverdun, J., Charroud, C., Le Bars, E., Molino, F., Menjot de Champfleur, S., et al. (2016). Cognitive Impairment and Basal Ganglia Functional Connectivity in Vascular Parkinsonism. AJNR. American Journal of Neuroradiology, 37(12), 2310–2316. https://doi.org/10.3174/ajnr.A4889.
Fartaria, M. J., Bonnier, G., Roche, A., Kober, T., Meuli, R., Rotzinger, D., et al. (2016). Automated detection of white matter and cortical lesions in early stages of multiple sclerosis. Journal of Magnetic Resonance Imaging, 43(6), 1445–1454. https://doi.org/10.1002/jmri.25095.
Gibson, E., Gao, F., Black, S. E., & Lobaugh, N. J. (2010). Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. Journal of Magnetic Resonance Imaging, 31(6), 1311–1322. https://doi.org/10.1002/jmri.22004.
Gonzalez-Redondo, R., Toledo, J., Clavero, P., Lamet, I., Garcia-Garcia, D., Garcia-Eulate, R., et al. (2012). The impact of silent vascular brain burden in cognitive impairment in Parkinson's disease. European Journal of Neurology, 19(8), 1100–1107. https://doi.org/10.1111/j.1468-1331.2012.03682.x.
Green, G. H., & Diggle, P. J. (2007). On the operational characteristics of the Benjamini and Hochberg False Discovery Rate procedure. Statistical Applications in Genetics and Molecular Biology, 6, Article27. https://doi.org/10.2202/1544-6115.1302.
Grober, E., Sanders, A. E., Hall, C., & Lipton, R. B. (2010). Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Disease and Associated Disorders, 24(3), 284–290. https://doi.org/10.1097/WAD.0b013e3181cfc78b.
Jones, J. D., Tanner, J. J., Okun, M., Price, C. C., & Bowers, D. (2017). Are Parkinson's Patients More Vulnerable to the Effects of Cardiovascular Risk: a Neuroimaging and Neuropsychological Study. Journal of the International Neuropsychological Society, 1–10. https://doi.org/10.1017/S1355617717000017.
Kandiah, N., Mak, E., Ng, A., Huang, S., Au, W. L., Sitoh, Y. Y., et al. (2013). Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism & Related Disorders, 19(7), 680–683. https://doi.org/10.1016/j.parkreldis.2013.03.008.
Kapeller, P., Barber, R., Vermeulen, R. J., Ader, H., Scheltens, P., Freidl, W., et al. (2003). Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke, 34(2), 441–445.
Lancaster, M. A., Seidenberg, M., Smith, J. C., Nielson, K. A., Woodard, J. L., Durgerian, S., et al. (2016). Diffusion Tensor Imaging Predictors of Episodic Memory Decline in Healthy Elders at Genetic Risk for Alzheimer's Disease. Journal of the International Neuropsychological Society, 22(10), 1005–1015. https://doi.org/10.1017/S1355617716000904.
Lee, S. J., Kim, J. S., Yoo, J. Y., Song, I. U., Kim, B. S., Jung, S. L., et al. (2010). Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Disease and Associated Disorders, 24(3), 227–233. https://doi.org/10.1097/WAD.0b013e3181d71a13.
Leung, I. H., Walton, C. C., Hallock, H., Lewis, S. J., Valenzuela, M., & Lampit, A. (2015). Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology, 85(21), 1843–1851. https://doi.org/10.1212/WNL.0000000000002145.
Litvan, I., Goldman, J. G., Troster, A. I., Schmand, B. A., Weintraub, D., Petersen, R. C., et al. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement Disorder Society Task Force guidelines. Movement Disorders, 27(3), 349–356. https://doi.org/10.1002/mds.24893.
Maillard, P., Carmichael, O., Fletcher, E., Reed, B., Mungas, D., & DeCarli, C. (2012). Coevolution of white matter hyperintensities and cognition in the elderly. Neurology, 79(5), 442–448. https://doi.org/10.1212/WNL.0b013e3182617136.
Mak, E., Dwyer, M. G., Ramasamy, D. P., Au, W. L., Tan, L. C., Zivadinov, R., et al. (2015). White Matter Hyperintensities and Mild Cognitive Impairment in Parkinson's Disease. Journal of Neuroimaging, 25(5), 754–760. https://doi.org/10.1111/jon.12230.
Malek, N., Lawton, M. A., Swallow, D. M., Grosset, K. A., Marrinan, S. L., Bajaj, N., et al. (2016). Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Movement Disorders, 31(10), 1518–1526. https://doi.org/10.1002/mds.26698.
Ng, A., Chew, I., Tay, K. Y., Au, W. L., Tan, L., & Kandiah, N. (2012). White matter disease adversely influences cognition in idiopathic Parkinson's disease. Alzheimer's & Dementia, 8, 171–172.
Nordahl, C. W., Ranganath, C., Yonelinas, A. P., Decarli, C., Fletcher, E., & Jagust, W. J. (2006). White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of Cognitive Neuroscience, 18(3), 418–429. https://doi.org/10.1162/089892906775990552.
Olsson, E., Klasson, N., Berge, J., Eckerstrom, C., Edman, A., Malmgren, H., et al. (2013). White matter lesion assessment in patients with cognitive impairment and healthy controls: reliability comparisons between visual rating, a manual, and an automatic volumetrical MRI method-the gothenburg MCI study. Journal of Aging Research, 2013, 198471. https://doi.org/10.1155/2013/198471.
Prins, N. D., van Straaten, E. C., van Dijk, E. J., Simoni, M., van Schijndel, R. A., Vrooman, H. A., et al. (2004). Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology, 62(9), 1533–1539.
Rektor, I., Bohnen, N. I., Korczyn, A. D., Gryb, V., Kumar, H., Kramberger, M. G., et al. (2018). An updated diagnostic approach to subtype definition of vascular parkinsonism - Recommendations from an expert working group. Parkinsonism & Related Disorders, 49, 9–16. https://doi.org/10.1016/j.parkreldis.2017.12.030.
Remy, F., Vayssiere, N., Saint-Aubert, L., Barbeau, E., & Pariente, J. (2015). White matter disruption at the prodromal stage of Alzheimer's disease: relationships with hippocampal atrophy and episodic memory performance. Neuroimage Clinical, 7, 482–492. https://doi.org/10.1016/j.nicl.2015.01.014.
Rodriguez-Oroz, M. C., Lage, P. M., Sanchez-Mut, J., Lamet, I., Pagonabarraga, J., Toledo, J. B., et al. (2009). Homocysteine and cognitive impairment in Parkinson's disease: a biochemical, neuroimaging, and genetic study. Movement Disorders, 24(10), 1437–1444. https://doi.org/10.1002/mds.22522.
Schmitter, D., Roche, A., Marechal, B., Ribes, D., Abdulkadir, A., Bach-Cuadra, M., et al. (2015). An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease. Neuroimage Clinical, 7, 7–17. https://doi.org/10.1016/j.nicl.2014.11.001.
Slawek, J., Wieczorek, D., Derejko, M., Dubaniewicz, M., Brockhuis, B., Sitek, E., et al. (2008). The influence of vascular risk factors and white matter hyperintensities on the degree of cognitive impairment in Parkinson's disease. Neurologia i Neurochirurgia Polska, 42(6), 505–512.
Slawek, J., Roszmann, A., Robowski, P., Dubaniewicz, M., Sitek, E. J., Honczarenko, K., et al. (2013). The impact of MRI white matter hyperintensities on dementia in Parkinson's disease in relation to the homocysteine level and other vascular risk factors. Neurodegenerative Diseases, 12(1), 1–12. https://doi.org/10.1159/000338610.
Smith, E. E., Salat, D. H., Jeng, J., McCreary, C. R., Fischl, B., Schmahmann, J. D., et al. (2011). Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology, 76(17), 1492–1499. https://doi.org/10.1212/WNL.0b013e318217e7c8.
Sunwoo, M. K., Jeon, S., Ham, J. H., Hong, J. Y., Lee, J. E., Lee, J. M., et al. (2014). The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with Parkinson's disease. European Journal of Neurology, 21(6), 922–e950. https://doi.org/10.1111/ene.12412.
Verger, A., Hossu, G., Kearney-Schwartz, A., Bracard, S., Roch, V., Van der Gucht, A., et al. (2016). Grey-matter metabolism in relation with white-matter lesions in older hypertensive patients with subjective memory complaints: a pilot voxel-based analysis study. Cerebrovascular Diseases, 42(1–2), 106–109. https://doi.org/10.1159/000445527.
Vesely, B., & Rektor, I. (2016). The contribution of white matter lesions (WML) to Parkinson's disease cognitive impairment symptoms: a critical review of the literature. Parkinsonism & Related Disorders, 22(Suppl 1), S166–S170. https://doi.org/10.1016/j.parkreldis.2015.09.019.
Zhou, Y., Yu, F., Duong, T. Q., & Alzheimer's Disease Neuroimaging, I. (2015). White matter lesion load is associated with resting state functional MRI activity and amyloid PET but not FDG in mild cognitive impairment and early Alzheimer's disease patients. Journal of Magnetic Resonance Imaging, 41(1), 102–109. https://doi.org/10.1002/jmri.24550.
Funding
Financial support was received from the Montpellier University Hospital AOI PARKIMAGE (N°ID-RCB2011-A00693–38). The funding source had no involvement in the conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Vincent Dunet, Mario Joao Fartaria, Jeremy Deverdun, Emmanuelle Le Bars, Florence Maury, Giovanni Castelnovo, Christian Geny and Nicolas Menjot de Champfleur do not declare any conflict of interest. Tobias Kober and Benedicte Marechal are employees of Siemens Healthcare Switzerland. Meritxell Bach Cuadra is supported by the Centre d’Imagerie BioMédicale (CIBM) of the University of Lausanne (UNIL), the Ecole Polytechnique Fédérale de Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG), and the Leenaards and the Jeantet Foundations.
Research involving human participants
This study was approved by the Institution Ethics Committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All participants gave their informed consent prior to their inclusion in the study. There are no details in this manuscript that might disclose the identity of the participants.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dunet, V., Fartaria, M.J., Deverdun, J. et al. Episodic memory decline in Parkinson’ s disease: relation with white matter hyperintense lesions and influence of quantification method. Brain Imaging and Behavior 13, 810–818 (2019). https://doi.org/10.1007/s11682-018-9909-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9909-x