Abstract
Objectives
To investigate the volumetric alterations of hippocampal subfields and identify which subfields contribute to mild cognitive impairment (MCI) in multiple system atrophy (MSA) and Parkinson’s disease (PD).
Methods
Thirty MSA-MCI, 26 PD-MCI, and 30 healthy controls were administered cognitive assessment, along with hippocampal segmentation using FreeSurfer 6.0 after a 3-T MRI scan. Regression analyses were performed between the volumes of hippocampal subfields and cognitive variables.
Results
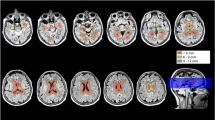
Compared with healthy controls, the volume of the hippocampal fissure was enlarged in PD-MCI patients, while left Cornu Ammonis (CA2–CA3), bilateral molecular layer, bilateral hippocampus–amygdala transition area, right subiculum, right CA1, right presubiculum, right parasubiculum, and bilateral whole hippocampus were reduced in the MSA-MCI group. Moreover, volumetric reductions of the bilateral hippocampal tail, bilateral CA1, bilateral presubiculum, bilateral molecular layer, left CA2–CA3, left hippocampus–amygdala transition area, right parasubiculum, and bilateral whole hippocampus were found in MSA-MCI relative to the PD-MCI group. The volumes of the left CA2–CA3 (B = − 11.34, p = 0.006) and left parasubiculum (B = 4.63, p = 0.01) were respectively correlated with language and abstraction functions. The volumes of the left fimbria (B = 6.99, p = 0.002) and left hippocampus–amygdala transition area (B = 2.28, p = 0.009) were correlated with visuospatial/executive function.
Conclusions
The MSA-MCI patients showed more widespread impairment of hippocampal subfields compared with the PD-MCI group, involving trisynaptic loop and amygdala–hippocampus interactions. The alteration of CA, hippocampus–amygdala transition area, and fimbria still requires further comparison between the two patient groups.
Key Points
• The atrophy patterns of hippocampal subfields differed between MSA and PD patients.
• MSA has widespread change in trisynaptic loop and amygdala–hippocampus interactions.
• The atrophy patterns may help to understand the differences of cognitive impairment in MSA and PD.

Similar content being viewed by others
Abbreviations
- CA:
-
Cornu Ammonis
- H-Y:
-
Hoehn and Yahr
- HC:
-
Healthy controls
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- MPRAGE:
-
Magnetization-prepared rapid acquisition gradient echo
- MSA-MCI:
-
MSA patients with MCI
- MSA:
-
Multiple system atrophy
- PD-MCI:
-
PD patients with MCI
- PD-NCI:
-
PD patients with no CI
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- UPDRS-III:
-
The Unified Parkinson’s Disease Rating Scale
References
Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12:133–147
Wang PS, Wu HM, Lin CP, Soong BW (2011) Use of diffusion tensor imaging to identify similarities and differences between cerebellar and Parkinsonism forms of multiple system atrophy. Neuroradiology 53:471–481
Stankovic I, Krismer F, Jesic A et al (2014) Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord 29(7):857–867
Foo H, Mak E, Chander RJ et al (2017) Associations of hippocampal subfields in the progression of cognitive decline related to Parkinson’s disease. Neuroimage Clin 14:37–42
Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA (2000) Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 3:1149–1152
Brenneis C, Egger K, Scherfler C et al (2007) Progression of brain atrophy in multiple system atrophy: a longitudinal VBM study. J Neurol 254(2):191–196
Danti S, Toschi N, Diciotti S et al (2015) Cortical thickness in de novo patients with Parkinson disease and mild cognitive impairment with consideration of clinical phenotype and motor laterality. Eur J Neurol 22(12):1564–1572
Xu Y, Yang J, Hu X, Shang H (2016) Voxel-based meta-analysis of gray matter volume reductions associated with cognitive impairment in Parkinson’s disease. J Neurol 263(6):1178–1187
Kandiah N, Zainal NH, Narasimhalu K et al (2014) Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat Disord 20(11):1203–1208
Asi YT, Ling H, Ahmed Z, Lees AJ, Revesz T, Holton JL (2014) Neuropathological features of multiple system atrophy with cognitive impairment. Mov Disord 29(7):884–888
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601
de Flores R, La Joie R, Chételat G (2015) Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 309:29–50
Pereira JB, Junqué C, Bartrés-Faz D, Ramírez-Ruiz B, Marti MJ, Tolosa E (2013) Regional vulnerability of hippocampal subfields and memory deficits in Parkinson’s disease. Hippocampus 23(8):720–728
Beyer MK, Bronnick KS, Hwang KS et al (2013) Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson’s diease. J Neurol Neurosurg Psychiatry 84:23–28
Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK, Gentleman SM (2009) Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord 15:196–204
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Koga S, Parks A, Uitti RJ et al (2017) Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov Disord 32(3):405–413
Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT (2015) Diagnosing PD-MCI by MDS Task Force criteria: how many and which neuropsychological tests. Mov Disord 30(3):402–406
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055
Iglesias JE, Augustinack JC, Nguyen K et al (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137
Messina D, Cerasa A, Condino F et al (2011) Patterns of brain atrophy in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord 17(3):172–176
Cykowski MD, Coon EA, Powell SZ et al (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138(Pt 8):2293–2309
Kovari E, Gold G, Herrmann FR et al (2003) Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106:83–88
Homma T, Mochizuki Y, Komori T, Isozaki E (2016) Frequent globular neuronal cytoplasmic inclusions in the medial temporal region as a possible characteristic feature in multiple system atrophy with dementia. Neuropathology 36(5):421–431
Churchyard A, Lees AJ (1997) The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson’s disease. Neurology 49:1570–1576
Yang W, Yu S (2017) Synucleinopathies: common features and hippocampal manifestations. Cell Mol Life Sci 74(8):1485–1501
Gertje EC, Pluta J, Das S et al (2016) Clinical application of automatic segmentation of medial temporal lobe subregions in prodromal and dementia-level Alzheimer’s disease. J Alzheimers Dis 54(3):1027–1037
Bastos-Leite AJ, van Waesberghe JH, Oen AL, van der Flier WM, Scheltens P, Barkhof F (2006) Hippocampal sulcus width and cavities: comparison between patients with Alzheimer disease and nondemented elderly subjects. AJNR Am J Neuroradiol 27:2141–2145
Armstrong RA, Kotzbauer PT, Perlmutter JS et al (2014) A quantitative study of alpha-synuclein pathology in fifteen cases of dementia associated with Parkinson disease. J Neural Transm (Vienna) 121:171–181
Basu J, Siegelbaum SA (2015) The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb Perspect Biol 7(11):1–26
Lisman JE (1999) Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 22(2):233–242
Jones MW, McHugh TJ (2011) Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci 34(10):526–535
Chevaleyre V, Piskorowski RA (2016) Hippocampal area CA2: an overlooked but promising therapeutic target. Trends Mol Med 22(8):645–655
Santos-Filho C, de Lima CM, Fôro et al (2014) Visuospatial learning and memory in the Cebus apella and microglial morphology in the molecular layer of the dentate gyrus and CA1 lacunosum molecular layer. J Chem Neuroanat 61-62:176–188
Mak E, Gabel S, Su L et al (2017) Multi-modal MRI investigation of volumetric and microstructural changes in the hippocampus and its subfields in mild cognitive impairment, Alzheimer’s disease, and dementia with Lewy bodies. Int Psychogeriatr 29(4):545–555
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. GuoGuang Fan.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. XiaoGuang Luo kindly provided statistical advice for this manuscript.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 599 kb)
Rights and permissions
About this article
Cite this article
Wang, N., Zhang, L., Yang, H. et al. Do multiple system atrophy and Parkinson’s disease show distinct patterns of volumetric alterations across hippocampal subfields? An exploratory study. Eur Radiol 29, 4948–4956 (2019). https://doi.org/10.1007/s00330-019-06043-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06043-9