Abstract
Ontogenetic aging of tissues and the gradual decrease of adventitious rooting are known challenges for the clonal propagation of woody species, hampering clonal forestry programs. This study examined possible signatures of tissue rejuvenation/reinvigoration in different propagated materials of Eucalyptus microcorys by analyzing the total protein profile, peroxidase activity, macro- and micronutrient contents, and adventitious rooting of mini cuttings. The analyses were performed on E. microcorys shoots which were successfully obtained by seminal and grafting propagation, micropropagation with epicormic shoots, and indirect organogenesis. Among four mature trees used in the propagation, tissues from the one with the best propagation results were investigated for signs of tissue rejuvenation and/or reinvigoration. Five individuals from each technique were randomly selected and transferred to a semi-hydroponic “channel” system. After four weeks in the seedbed, the total protein, peroxidase activity, nutrient content and rooting of the mini cuttings were evaluated. SDS-PAGE enabled the differentiation of leaf samples obtained by grafting from the other propagation techniques, as revealed by two distinct bands. Materials obtained by micropropagation with epicormic shoots showed the highest peroxidase activity, while those obtained by seminal propagation and from the selected mature tree showed the lowest peroxidase activity. A portable X-ray fluorescence spectroscope (pXRF) identified adequate nutrient content in most of the nutrients tested in materials obtained by seminal and grafting propagation, and by indirect organogenesis. The analysis of adventitious rooting showed that the highest rooting percentage was observed in mini cuttings from seminal propagation (75%) followed by indirect organogenesis (35%). Based on principal component analysis, it was concluded that rooting of mini cuttings from both seminal propagation and indirect organogenesis was associated with phosphorous, sulphur, and potassium contents, which suggests a higher level of tissue rejuvenation/reinvigoration in these propagated plants. Further studies are recommended to search for other methods that present similarities with the responses to adventitious rooting in forest species and thus optimize the rescue and propagation of plants with distinct ontogenetic stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most perennial woody species undergo changes in morphology, physiology, and biochemical processes during the transition from juvenile to adult stages. Determining the ontogenetic age of tissues, although still a challenge in clonal forestry, is required for successful clonal propagation, as the desired characteristics of the adult tree are expressed at maturity (Mercado et al. 2021; Santos Junior et al. 2021). Adult plants gradually decrease their rooting ability towards more advanced ontogenetic stages. Mature branches tend to have lower concentrations of auxin, increase in rooting inhibitors and in phenolic levels, in addition to an anatomical barrier of lignified tissue between the phloem and the cortex (Xavier et al. 2021). Characteristics related to the maturation of plant tissues can be stable and/or reversible and may vary depending on the level of juvenility. Plant tissues may revert from an adult to a juvenile stage of high physiological vigour without necessarily reducing ontogenetic age through rejuvenation/reinvigoration techniques (Wendling et al. 2014). Serial grafting, micropropagation, and rooting of cuttings (i.e., repeated propagation) are common propagation methods to induce growth of juvenile shoots in perennial woody species and to increase the production of propagules prone to adventitious rooting (Xavier et al. 2021).
Evaluating the suitability of propagules for adventitious rooting is particularly useful in studying how propagation techniques perform on the reinvigoration and rejuvenation of plant tissues. Total protein electrophoresis can be used to predict the biochemical changes that induce adventitious rooting, especially in plant materials of different ontogenetic ages (Han et al. 2014; Zhang et al. 2015). Analysis at the protein level allows broad insight into the functional interactions of different metabolic pathways. The method based on the association of trichloroacetic acid (TCA) with acetone enables fast extraction times with high yields of high-quality protein at low cost, providing satisfactory results for materials from woody species (Zhang et al. 2015).
In more specific routes during the adventitious rooting stage, there is a significant increase in the activity of peroxidases, enzymes involved in the metabolism of phenolic compounds (hydrogen peroxide; H2O2), acting in the regulatory process of root formation (Rodríguez et al. 2020; Gong et al. 2022). Differences in accumulation between superoxide radicals and H2O2 significantly affect root growth and differentiation (Dunand et al. 2007), as shown with mini cuttings of Eucalyptus grandis × E. urophylla (Prado et al. 2014) and in the propagation of several semi-, non-, and woody plants, for instance, Linum usitatissimum L. (Takáč et al. 2016) and Cordia trichotoma (Vell.) Arrab. ex. Steud. (Silva et al. 2022).
Although the importance of nutrients in the adventitious rooting process is recognized, for example phosphorous (P), sulphur (S), potassium (K), calcium (Ca), zinc (Zn), they are poorly understood due to the biochemical and physiological complexity during rhizogenesis (Cunha et al. 2009a, b; Brondani et al. 2014; Hartmann et al. 2017). Portable X-ray fluorescence spectroscopy (pXRF) offers an effective way of quantifying or qualifying virtually any element quickly and non-destructively, in the field or in the laboratory (McGladdery et al. 2018). As each element has a specific spectral signature and its energy intensity is proportional to the concentration of the element in the material, the specific energy value is used for element identification, whereas the fluorescence intensity is used for quantification (Weindorf et al. 2014).
While progeny and provenance tests are important tools to select genotypes of interest to the final product, experimental old-growth forests are rare. However, studies using species of the genera Eucalyptus and Corymbia have been carried out since 1974 at an old-growth experimental site in Lavras, Minas Gerais, Brazil. The site is managed by the Universidade Federal de Lavras (UFLA) (IPEF 1984). Among tree species, Eucalyptus microcorys F. Muell. has characteristics that are favourable to silvicultural applications, such as moderate resistance to frost, drought-resistant species, tolerance to fire, and good regeneration capacity by stump coppicing (Atala et al. 2022). Despite its potential commercial applications, E. microcorys has not been established commercially nor it has been studied as often as other Eucalyptus species. Moreover, existing studies on E. microcorys are mainly limited to its adaptation and wood utilization aspects (Oliveira et al. 2014; Teixeira et al. 2020).
Based on the role of research in improving methods for efficient propagule rooting and consequently facilitating the rescue of materials with high ontogenetic age, especially in woody species, this study investigated the usefulness of tests for total proteins, peroxidase activity, nutrient quantification by a portable X-ray fluorescence spectroscope (pXRF), and adventitious rooting of mini cuttings in determining the occurrence of tissue rejuvenation/reinvigoration in propagated plants of E. microcorys.
Materials and methods
Source of tissues
Tissues were collected from selected mature trees of over 44-years-old E. microcorys in an experimental forest established in 1974, containing different provenances of Eucalyptus and Corymbia, at the forest nursery managed by UFLA in Lavras, Minas Gerais (21°22′75'' S, 44°96′98'' W).
Vegetative propagation techniques
Seven propagation techniques of a non-destructive nature, namely seminal propagation, propagation by cuttings, propagation by air layering, grafting propagation, micropropagation with annual shoots, micropropagation with epicormic shoots, and micropropagation via indirect organogenesis, were carried out using propagules from four mature individuals of E. microcorys (Fig. 1).
Seminal propagation
Seeds were collected from selected trees and sown in 110 cm3 tubes filled with vermiculite and organic material (1:1, v/v). The tubes were kept in polypropylene trays in beds 80 cm above the ground in a shade house with 50% of natural light. Sprinkler irrigation occurred twice in the morning and in the afternoon, except on rainy days. After 30 days, the seedlings were placed in direct sunlight. Thinning was performed with scissors, leaving in the tube only the most centralized seedling with better shoot growth. After 120 days, survival and hardening were evaluated in 60 plants per selected tree, using two samples.
Propagation by cuttings
Cuttings from lateral shoots of the last vegetative period were collected from the selected trees and standardized at a 3-cm length with a pair of leaves reduced by half. Cuttings were treated with 1 mg mL−1 of indole-3-butyric acid (IBA) at their base for 15 s and kept in 110 cm3 tubes containing vermiculite and organic material (1:1, v/v), stored in a greenhouse under controlled relative humidity (> 80%) and temperature (20–35 °C) maintained by an intermittent misting system with high pressure and low flow nozzles, and automatically controlled by a humidistat. After 90 days in the greenhouse, survival and adventitious rooting were evaluated in 60 cuttings per selected tree, using two samples.
Propagation by air layering
Air layering was carried out directly on the selected trees using 1–2 cm diameter branches from the latest vegetative period. Half of the bark of each branch was removed with a budding knife, forming a 1.0–1.5 cm wide semi-ring, covered with a substrate containing vermiculite and organic material (1:1, v/v) and sealed with a 10 cm × 20 cm transparent plastic film to retain moisture. After 150 days in the field, survival and adventitious rooting were evaluated in 20 air layers per tree.
Grafting propagation
Seminal seedlings from the selected trees were produced in 110 cm3 tubes and, after 150 days, used as rhizome material. The full slit grafts were obtained from seasonal shoots from the selected trees. Cuts were made using grafting pliers and the tissues unified with 2-mm wide micropore tape. The grafted seedlings were placed in a heated greenhouse with controlled relative humidity (> 80%) and temperatures (20–35 °C) maintained by an intermittent misting system with high pressure and low flow nozzles, and automatically controlled by a humidistat. After 45 days, tissue attachment and emission of new shoots were evaluated in 60 grafted plants per tree, sampled twice.
Micropropagation with seasonal shoots
Tissues were collected directly from the selected trees and the explants standardized using nodal segments 1.0–1.5 cm long from the median portion of the epicormic shoots comprising axillary buds and leaves removed. The explants were superficially sanitized for 10 min in running water, followed by 5 min in 50% sodium hypochlorite (NaOCl; water: hypochlorite, v/v, 2.0–2.5% active chlorine) and then placed in a laminar flow chamber, washed three times with deionized and autoclaved water. The explants were inoculated in 2.5 cm × 15.0 cm test tubes containing 10 mL of MS culture medium (Murashige and Skoog 1962) without plant growth regulators and kept in a growth room. The preparation of the culture medium and the environmental conditions in the growth room were as described by Faria et al. (2021). The methodology was evaluated in 60 explants per sample, first after 30 days of in vitro inoculation and then 460 days later until ex vitro rescue of the materials.
Micropropagation with epicormic shoots
Epicormic shoots were obtained from branches collected from the selected trees. The branches were conditioned in a heated greenhouse with controlled relative humidity (> 80%) and temperatures (20–35 °C) maintained by an intermittent high pressure misting system and low flow nozzles automatically controlled by a humidistat. After 45 days, the epicormic shoots were collected to obtain the explants and were standardized using 1.0–1.5 long nodal segments from the median portion of the epicormic shoots containing axillary buds and with leaves removed. The explants underwent asepsis for 10 min in running water, followed by 5 min immersion in 50% sodium hypochlorite (NaOCl) (water: hypochlorite, v/v, 2.0–2.5% of active chlorine), and then placed in a laminar flow chamber and washed three times with deionized and autoclaved water. Afterwards, the explants were inoculated in test tubes containing 10 mL of MS culture medium without plant growth regulators and kept in a growth chamber. The preparation of the culture medium and the environmental conditions in the growth room were as described by Faria et al. (2021). After 30 days of in vitro inoculation, 60 explants per sample were evaluated, and again after 460 days until ex vitro rescue of the materials.
Micropropagation via indirect organogenesis
Explants were obtained from E. microcorys seedlings by in vitro germination. Asepsis was performed by washing the seeds in running water for five minutes, and then stirred for 15 min in a solution of sodium hypochlorite—NaOCl (2.0– 2.5% of active chlorine) with three drops of detergent (0.05 mL). The seeds were washed three times with distilled and autoclaved water in a laminar flow chamber and inoculated in an in vitro culture medium glass test tubes containing 10 mL of MS culture medium consisting of only distilled water and agar. Germination occurred within 12 days and established within 30 days of in vitro inoculation. After establishment, 0.5 cm hypocotyl segments were collected and inoculated into circular glass flasks containing 40 mL of WPM culture medium (Lloyd and McCown 1980) supplemented with 1 mg L−1 of thidiazuron (TDZ) (Sigma, Belo Horizonte, Brazil) + 4 mg L−1 of α-naphthaleneacetic acid (NAA) (Sigma, Belo Horizonte, Brazil). After inoculation, the explants were kept in a dark growth chamber for 60 days, and subcultured after 30 days according to Faria et al. (2021). After 60 days, the occurrence of callogenesis in 60 explants per sample was evaluated, and then after 305 days until ex vitro rescue of the materials.
Investigation of tissue rejuvenation and reinvigoration
Among the four mature trees, tissues from the one with the best propagation results were investigated for signs of rejuvenation and/or reinvigoration. Five individuals from each technique were randomly picked and transferred to a semi-hydroponic “channel” system. After four weeks in this seedbed, total protein, peroxidase activity, nutrient content and rooting of the mini cuttings were evaluated.
Total protein extraction–TCA/acetone/phenol—and SDS-PAGE profiling
Young leaves were used for each propagated material, with four replicates for each propagation source. The samples were ground in a porcelain crucible using liquid nitrogen. A total of 200 mg of leaf powder were used for extraction in 1.5 mL of 10% TCA in acetone, homogenized in a vortex and centrifuged at 14,000 rpm for 3 min at 4 °C to obtain the precipitate. The resulting pellet was washed twice, first, with 1.5 mL of 80% methanol containing 0.1 M of ammonium acetate and then with 1.5 mL of 80% acetone, centrifuging between washes at 14,000 rpm for 3 min at 4 °C. The pellet was oven dried at 50 °C for 10 min, homogenized in 0.5 mL of phenol (pH 8.0) and 0.5 mL of SDS buffer (30% sucrose, 2% SDS, 0.1 Tris–HCl pH 8.0, 5% 2-β-mercaptoethanol), incubated for 5 min and then centrifuged for 3 min at 4 °C. The resulting supernatant was transferred to new test tubes containing 1.5 mL of methanol with 0.1 M of ammonium acetate and incubated at − 20 °C for 10 min. The samples were then centrifuged for 5 min at 4 °C. The pellets were finally washed with 100% methanol and 80% acetone and resuspended in urea buffer.
A small fraction of each sample was used for protein quantification following Bradford (1976). Samples of 50 µL were applied in wells of a discontinuous SDS gel, (the concentrator phase at 6.0% and separator phase at 7.5% of acrylamide), in two technical replications. The set was immersed in 1 × SDS running buffer (0.052 M tris base solution, 0.053 M glycine, and 0.0035 M SDS), to which a current of 150 V was applied for seven hours. After electrophoresis, the gels were fixed for 30 min in a solution of 7% acetic acid (v/v) and 40% methanol (v/v), and transferred to a staining solution containing 0.1% (w/v) Coommassie Brilliant Blue G-250, 1.6% (v/v) phosphoric acid, 12% (w/v) ammonium sulphate, and methanol 20% (v/v) for 24 h. At the end of this period, the gels were decolorized in a 10% acetic acid and 5% ethanol solution for 5 h and scanned (Imagescanner, Amersham Biosciences, UK). The molecular weight marker Kaleidoscope® Precision Plus Protein Standards was used.
Peroxidase activity
Young leaves were used for each propagated material, with five replicates. A total of 200 mg of fresh samples were ground in liquid nitrogen with 50% of PVPP (polyvinylpyrrolidone). A 1.5 mL extraction buffer (400 mM aqueous potassium phosphate, pH 7.8; 10 mM EDTA (ethylenediaminetetraacetic acid); 200 mM ascorbic acid; and water) was added. The test tubes with the mixture was centrifuged at 8,500 rpm for 10 min at 4 °C, and the supernatants collected and refrigerated for 24 h. To quantify the peroxidase activity, the methodology of Nakano and Asada (1981) was used with modifications by García-Limones et al. (2002). The samples were distributed on an ELISA plate reader, with 16 µL of the sample pipetted in triplicate. A total of 154 µL of the reaction mix (200 mM aqueous potassium phosphate solution, pH 6.5; 200 mM guaiacol and water) was used. Before the readings, 30 µL of H2O2 (0.35%) were added. Peroxidase activity readings were performed using a spectrophotometer with a biotek epoch microplate reader configured for readings at 470 nm connected to a computer, and Gen5 software used to record the readings. Sixty readings were performed at 1-min intervals, totalling 60 min to record peroxidase activity in the samples.
Nutrient quantification via pXRF
Portable X-ray florescent spectroscope readings were performed by scanning a set of five young leaves for each propagated material. Before digitizing, the instrument was heated for 30 min and the leaf samples folded in half and secured at the ends with rubber bands. The digitalization of each sample was performed with a DP-6000 spectrometer (Olympus, Waltham, MA, USA) configured in the equipment's Solo Mode (Weindorf and Chakraborty 2020). The samples were arranged in X-rays operated at 10–40 keV with a sequence of three beams per reading lasting 30 s each, so that a complete reading lasted 90 s. Each treatment was performed in duplicate with the average for each computed at the end. To validate the instrument's performance, reference materials certified by the Brazilian National Institute of Standards and Technology were used to establish the correction factor for each nutrient applied to the dataset (Koch et al. 2017). Table 1 shows the macro and micronutrients contents [phosphorus (P), potassium (K), calcium (Ca), sulphur (S), zinc (Zn), iron (Fe), manganese (Mn), and silicon (Si)] considered adequate, high, low, and deficient for Eucalyptus shoots in a mini-clonal garden environment. These values were used as a reference to the pXRF readings according to Higashi et al. (2004).
Adventitious rooting test
Two lots of 24 replicates of mini cuttings from lateral shoots were standardized with an average size of 3 cm with a pair of leaves reduced by half. This was conducted in 110 cm3 tubes containing vermiculite and organic material (1:1, v/v), stored in a heated greenhouse with controlled humidity (> 80%) and temperature (20–35 °C). After 60 days in the greenhouse, the percent rooting was evaluated.
Statistical analysis
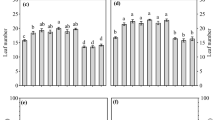
Data on peroxidase activity were submitted to the Hartley (P > 0.05) and Shapiro–Wilk (P > 0.05) tests, and analysis of variance (ANOVA, P < 0.05) was subsequently performed. According to the significance of the F test, the groups were compared using Tukey’s test (P < 0.05). Principal component analysis (PCA) assessed the existence of gradients between the treatments as well as to analyze the most important variables. An accumulated variance greater than 80% was used as a criterion for selecting the number of principal components.
Results
Vegetative propagation techniques
Cuttings, air layering, and micropropagation techniques with annual shoots were not suitable for cloning the selected material as there was total mortality (Fig. 2 A–F). Propagation by grafting, micropropagation with epicormic shoots, micropropagation with indirect organogenesis, and via seminal pathway satisfactorily produced new shoots, resulting in 10%, 40%, 73%, and 90% success, respectively (Fig. 2 G–L). These techniques were therefore selected to investigate indicators of tissue rejuvenation and/or reinvigoration of E. microcorys.
Overview of the propagation techniques as follows: A standard form used in the cutting technique; B propagation failure, indicating mortality after 90 days in a greenhouse (bar = 3 cm); C standard form of air layering; D rescue failure, indicating drought and mortality after 150 days in the field (bar = 6 cm); E standard form of explants in vitro culture; F rescue failure, indicating contaminated explant after 30 days in vitro (bar = 1 cm); G success in propagation via grafting technique, showing new shoots in the graft (bar = 6 cm); H standard form of epicormic shoots used as explants in the micropropagation technique (bar = 2 cm); I explant from epicormic shoots cultivated in vitro during the bud multiplication stage (bar = 1 cm); J callus regenerated via indirect organogenesis from the hypocotyl tissue of seedlings in vitro (bar = 1 cm); K standard form of plants acclimatized via micropropagation with epicormic shoots and via indirect organogenesis, after 490 and 395 days under in vitro culture (bar = 1 cm); L production of seedlings via seminal route after 120 days of germination
TSDS-PAGE protein profiling
Analysis of total proteins by electrophoresis showed a similar pattern of protein bands extracted with the TCA/Acetone/Phenol method in the different propagation techniques tested in this study. The separation of proteins by molecular weight was efficient, resulting in numerous bands between 100 and 25 kD (kilodaltons) regions (Fig. 3). There was generally a greater number of bands between 75 and 37 kD regions in all propagation techniques. The presence of two bands with a distinct pattern in propagation via grafting compared to the other techniques was observed, with one between 100 and 75 kD and the other between 37 and 25 kD (Fig. 3).
Total protein electrophoresis profile by the TCA/acetone/phenol method in leaf samples obtained through different propagation techniques of Eucalyptus microcorys; Samples: 1. selected tree; 2. seminal route; 3. via grafting; 4. via micropropagation (epicormic shoots); 5. via micropropagation (indirect organogenesis); arrows indicate the two distinct regions in sample 3. n = four replicates for each material source
Peroxidase activity
This differed significantly among the propagation techniques in this study. Individuals micro propagated with epicormic shoots showed higher levels peroxidase activity than those obtained with the other propagation strategies. Individuals propagated with grafting had the second highest mean peroxidase activity, while the lowest mean activities were observed in samples of the selected tree and seedlings from the seminal propagation (Table 2).
Nutrient quantification via portable X-ray florescence spectroscopy (pXRF)
According to the readings of the element contents in the propagated E. microcorys materials by pXRF, P, K, Ca, S, Zn, Fe, Mn, and Si contents were analyzed (Table 3). Phosphorous levels varied between propagation methods, with seedlings from the seminal propagation and from indirect organogenesis having the highest mean. Individuals propagated by micropropagation with epicormic shoots had the lowest average, with values closest to the selected tree. Potassium had the smallest variation between the propagation techniques, ranging from 16.6 to 17.8 g kg−1, while in the selected tree it was nearly half (Table 3). The highest average calcium was in the selected tree (14 g kg−1), while the lowest was in materials propagated via grafting and seminal routes. Sulphur levels varied between 1.4 and 2.5 g kg−1, with individuals from seminal propagation and from indirect organogenesis having the highest means (Table 3).
With regards to micronutrients, Zn content differed considerably among materials from each propagation technique and the selected tree. Grafting resulted in materials with the highest mean Zn content (52.5 mg kg−1), while the selected tree had the lowest mean Zn content (19.5 mg kg−1). Fe levels ranged from 104 to 305 mg kg−1 in the propagated materials, while in the selected tree the mean Fe content was nearly twofold higher than in material propagated by grafting. Mn content largely varied between differently propagated materials and the selected tree. While the selected tree had the highest absolute mean (1,487 mg kg−1), the second highest Mn content was found in materials from the seminal propagation (286 mg kg−1); the other materials had Mn contents close to 143 mg kg−1. Regarding Si, the readings recorded between 841 and 1,048 mg kg−1, with the selected tree having the highest Si content (Table 3).
Adventitious rooting test
To determine the percentage of adventitious rooting of mini cuttings, an experiment was carried out in the greenhouse for 60 days. High variation was detected, with results ranging from zero to 75.0% of rooting (Table 4). The highest mean rooting of mini cuttings (75.0%) resulted from material propagated by seedlings through the seminal route (corresponding to eighteen materials), and from material propagated via micropropagation through indirect organogenesis, with 33.3% of mini cuttings rooted (corresponding to eight materials). Individuals micropropagated by epicormic shoots and by grafting had only 4.2% of materials with roots (corresponding to one rooted mini cutting), while those collected directly from the selected tree had no root formation.
Principal component analysis
PCA distinguished between the different tests to verify the degree of tissue rejuvenation and/or reinvigoration. There was a trend to a subdivision of materials into three groups: the first from the percentage of rooting of mini cuttings and nutrients P and S; the second by peroxidase activity and K and Zn nutrients; and the third by the nutrients Ca, Fe, Mn, and Si (Fig. 4). Through these groupings it was also possible to assess the differences of the materials propagated, with a group formed by individuals via indirect organogenesis and seminal route (group 1–materials come from juvenile tissues), another group formed by individuals via grafting and via micropropagation through epicormic shoots (group 2–materials of clonal origin with propagules from the selected tree) and one more distant through the selected tree (group 3–material come from mature tissues) (Fig. 4)..
Discussion
Among the techniques used to obtain tissue rejuvenation and/or reinvigoration in E. microcorys, it was possible to verify a gradient: via micropropagation through epicormic shoots < via grafting < via micropropagation through indirect organogenesis < via seminal propagation. Based on the tests carried out (total protein tests, peroxidase activity, readings by pXRF and the mini cutting rooting test), seminal propagation and micropropagation through indirect organogenesis had the strongest relationship with rooting of propagules. This result confirmed our hypothesis, as these two materials come from physiologically younger tissues (e.g., seeds). For some woody species, cuttings from young seedlings derived from seeds root easily, while ones originating from older plants root sporadically or not at all (Wendling et al. 2014).
SDS-PAGE profiling of total protein content
Total proteins (TCA/acetone/phenol) showed a differentiated behaviour of the protein profile in leaf tissue samples via grafting. Propagation by grafting is a complex process and may introduce several variations in tissues which influence the flow of essential mineral elements, the growth of grafted tissues with the rhizome and the development of parenchymal or bark tissue, in addition to genetic factors of the materials (Barbosa et al. 2016; Hartmann et al. 2017). The effect of grafting on the protein profile of seedlings of Carya cathayensis Sarg., Lagenaria siceraria (Molina) Standl. var. dayehuzi, and of the latter with Citrullus lanatus (Thumb) Matsum. and Nakai have been reported (Song et al. 2016; Wang et al. 2016; Xu et al. 2017). In this study, the material used for grafting was in a much more advanced ontogenetic stage than the rootstock, which had juvenile ontogenetic characteristics. These characteristics of the grafting technique may have contributed to the formation of other metabolic routes compared to other propagation techniques for E. microcorys, which is a promising result for further studies within the limits of these regions in the protein profile, as the phenomenon is still undocumented.
Peroxidase activity
The highest peroxidase activity was in materials from micropropagation with epicormic shoots, followed by grafted materials. Peroxidase activity is a biochemical indicator related to adventitious root development. Studies have reported that peroxidase activity varies with auxin levels, with an increase in enzymatic activity observed during the root initiation stage (Kang et al. 2018; Wei et al. 2019). According to Aumond Jr et al. (2017), events associated with adventitious rooting in mature Eucalyptus globulus Labill showed an increase in peroxidase activity with loss of rooting ability. They associated the increase in peroxidase activity with a decrease in auxin activity, which are important plant growth regulators linked to adventitious rooting in cuttings. In this study, while a similar behaviour was observed with the different propagation techniques, peroxidase activity in the samples of the selected tree differed from the literature. It is noteworthy that, even with statistical differences in peroxidase activity, all propagation techniques carried out in this study resulted in a degree of tissue rejuvenation and/or reinvigoration.
Micropropagation has several advantages for the propagation of tree species, especially for ones that are difficult to root, whereas other techniques rarely succeed (Atala et al. 2022; Faria et al. 2022). Several studies recommend this propagation technique using epicormic shoots, mainly for species with high ontogenetic ages, for instance, for Eucalyptus benthamii Maiden & Cambage 13-years-old (Baccarin et al. 2015) and Eucalyptus cloeziana F. Muell 26-years-old (Oliveira et al. 2015). In this study, grafting resulted in the second highest mean peroxidase activity. Propagation by grafting is a complex process and contributes to several metabolic routes between the graft and the rhizome (Barbosa et al. 2016). Studies indicate that, due to the healing process at the grafted site, there is an accumulation of phenolic compounds that increase the activity of enzymes such as peroxidase, disturbing the vascular connections between the graft and rhizome, which hampers tissue compatibility (Pina et al. 2017; Baron et al. 2019). In this study, the percentage of grafted success was relatively low, largely attributed to the high ontogenetic age of the selected tree, with grafting being the only one that resulted in successful propagation compared to the other in vitro techniques.
Determination of nutrients by pXRF
The portable x-ray florescence spectroscopy was efficient to determine the different nutrients and contents in E. microcorys tissues. Generally, the nutrients with the best response to distinguish the propagated materials were P, S and K, indicating a direct relationship with adventitious rooting. Despite the importance of mineral nutrition in adventitious rooting, it is still an understudied topic for Eucalyptus species. Higashi et al. (2004) provides interpretations of nutrients for Eucalyptus shoots but it cannot be extrapolated to all genetic materials (i.e., non-commercial clones) nor to all nutrients (Cunha et al. 2009b). For our study, the use of the table proposed by Higashi et al. (2004) as a reference (see Table 1) contributed to the objective of verifying indications of tissue rejuvenation and/or reinvigoration of E. microcorys. Although the use of pXRF is aimed at elemental soil analysis, previous studies have demonstrated its effectiveness in assessing the elemental composition of leaf tissues and plant seeds (McGladdery et al. 2018; Zhou et al. 2020). In addition, this method has a high correlation (R2 > 0.90) for P, S, K, Ca, and Mn contents in leaves of various species with standard methods (Towett et al. 2016).
Through the analysis of macronutrients in materials of E. microcorys, differences among the materials were identified, mainly when compared to macronutrient contents in the selected tree. According to the reference values proposed by Higashi et al. (2004), none of the individuals propagated in this study had adequate levels of phosphorous. The best results for this nutrient were found in material propagated via the seminal route and micropropagated through indirect organogenesis, both with low levels of P. Among macronutrients, P is vital for cell differentiation, division, and multiplication, in the composition of carbohydrates, phospholipids that make up membranes, coenzymes, nucleoproteins, and nucleic acids, in addition to acting in the plant's vital processes due to the storage and transfer of energy in the form of adenosine triphosphate (ATP) (Pereira and Peres 2016). Moreover, studies often associate the low availability of P in plants with direct changes in the root system, particularly in their length and formation (Cunha et al. 2009a; Clausing et al. 2021). In addition to P, sulphur also contributes to adventitious rooting and is an essential mineral for regulating plant growth, interacting with auxins, supporting root induction and morphogenesis processes (Bouranis et al. 2020). In this study, sulphur was one of the nutrients most associated with adventitious rooting of mini cuttings. Sulphur contributes to several compounds and reactions in different plant metabolic pathways, being associated with increased photosynthesis and respiratory activity, increased protein synthesis, and decreased soluble carbohydrates (Pereira and Peres 2016; Narayan et al. 2022). Cunha et al. (2009b; c) observed that S levels considered deficient or low ensured satisfactory rooting of Eucalyptus spp. cuttings, indicating that, in this instance, the adventitious rooting process was little affected by S. In this study, S content had a positive relationship with rooting of E. microcorys mini cuttings, especially in materials propagated via seminal route and indirect organogenesis. These materials attained the highest percentages of adventitious rooting with adequate S content, according to the reference values in Higashi et al. (2004).
There was considerable similarity in potassium contents amongst the materials propagated in this study. All samples had above 16 g kg−1, which is considered adequate (Higashi et al. 2004). K is the second most nutrient in demand by plants as its main function is enzymatic activation, and its deficiency causes some chemical changes and a direct impact on the production of carbohydrates, an essential source of energy for root induction (Mateus et al. 2019; Sustr et al. 2019). In this study, only the selected tree was deficient in potassium. According to Hartmann et al. (2017), adequate levels of nutrients such as K and Ca are important for the processes of root formation. Calcium has a constituent role in photosynthesis, and in cytoplasmic movement in plant tissues and cell walls, ensuring the development of shoots and roots (Müller et al. 2017). Its deficiency can, therefore, interfere with the process of initiation and expression of the root (Soares et al. 2018). Higashi et al. (2000), in studies with Eucalyptus, concluded that Ca contents at adequate levels contribute to a higher percentage of clonal propagule rooting. In this study, the materials propagated by the two forms of micropropagation had adequate values of Ca, while ones propagated via grafting and the seminal route had low contents (approx. 3 g kg−1).
With regards to micronutrients, the micropropagated materials had low levels of Zn, while materials by grafting and seminal routes had adequate levels. The lowest mean value was observed in the selected tree, reflecting a deficiency of this micronutrient (Higashi et al. 2004). According to Hartmann et al. (2017), an increase in endogenous levels of indole-3-acetic acid (IAA), an auxin linked to adventitious rooting, may be favoured by the presence of Zn through its effect on increasing the production of tryptophan, a natural precursor of IAA and of reserve substances. Similar results relating zinc to an increase in adventitious rooting of mini cuttings were verified by Cunha et al. (2009c) with Eucalyptus grandis W. Hill ex Maiden and E. grandis × E. urophylla and by Brondani et al. (2014) with Eucalyptus benthamii Maiden & Cambage. With Fe, only the E. microcorys selected tree did not have adequate Fe levels. Iron is related to enzyme biosynthesis linked to peroxidase pathways, which are directly involved in cell growth and expansion, root differentiation and development, and auxin catabolism and lignification (Lima et al. 2018; Celletti et al. 2020). In this study, there was no similarity detected between peroxidase activity and Fe contents in individuals, especially when analyzing the adventitious rooting of mini cuttings.
Another important micronutrient involved in adventitious rooting is manganese. It promotes enzymes that oxidize IAA such as peroxidases (Cunha et al. 2009a). This role may be relevant, considering the different stages of rooting and the action of peroxidases. In Mn-deficient plants, IAA oxidase activity is high and causes the destruction of auxin, the main rooting-inducing hormone (Marschner 2012). In addition, plants deficient in Mn show a decrease in carbohydrates, the main source of energy for adventitious rhizogenesis (Vatansever et al. 2016). In this study, we found different Mn levels between samples of propagated materials. Seedlings propagated via the seminal route were the only ones that had adequate levels of Mn, while the other propagation techniques resulted in Mn-deficient materials. With silicon, all propagated materials had similar levels (approx. 850 mg kg−1), with only the selected tree showing inconsistent results. Si is not commonly used in the nutritional evaluation of plants, and there is no adequate recommendation in the literature to suggest adequate levels.
Adventitious rooting of mini cuttings
One of the most consistent expressions of maturation in woody plants has been the transition from high to low rooting capacity of stem and leaf cuttings (Wendling et al. 2014). Besides genetic material, some events, such as changing seasons and temperature fluctuations, may affect the adventitious rooting of mini cuttings, as observed in studies with Eucalyptus spp. (Gonin et al. 2019; Vilasboa et al. 2021; Azevedo et al. 2022). In this study, through the rooting test of mini cuttings propagated by different techniques, there was a large difference in rooting between the sources used, especially when compared to the selected tree. While the best outcomes for adventitious rooting of the mini cuttings were seedlings via the seminal route, there was also an intermediate degree of tissue rejuvenation and/or reinvigoration with the other techniques. Serial propagation by cuttings is a promising technique for reversing adult tissue in different species of Eucalyptus (Wendling and Xavier 2005). Mendonça et al. (2020) reported that in vitro rejuvenation/reinvigoration was a determining factor for greater rooting efficiency of mini cuttings for clonal rescue in Eucalyptus urophylla S.T. Blake. Therefore, even with only 5% rooting in mini cuttings, propagation techniques performed via grafting and via micropropagation through epicormic shoots are recommended for the propagation of E. microcorys with high ontogenetic age, with these two techniques being the only ones that used material of clonal origin with propagules from the selected tree.
The of observations in each propagation technique may be explained by principal component analyses. When analyzing axis 1 (F1), there was a clear separation between clusters, with the selected tree on the extreme right, while the propagation techniques are distributed on the extreme left. In addition, three clusters of materials were observed: the first related the percentage of rooting of mini cuttings and nutrients P and S with materials propagated via micropropagation through indirect organogenesis and seminal route; the second related to peroxidase activity and K and Zn nutrients with materials propagated by grafting and micropropagation through epicormic shoots; and the third related to the nutrients Ca, Fe, Mn and Si with materials from the selected tree (Fig. 4). These results demonstrate how the selected tree is distant from materials propagated using different techniques. One of the possible explanations could be the lower activity in metabolic pathways, and consequently the lower capacity in adventitious rooting of the mature plant. Therefore, based on the results obtained by tests for total protein, peroxidase activities, readings by pXRF, and the mini cutting rooting test, further work on this topic is required to seek other methods that have similarities with adventitious rooting responses in woody species. This would contribute to a better understanding of the propagation of materials with high ontogenetic age.
Conclusions
Plants replicated by grafting show a differential pattern of protein bands analyzed by SDS/PAGE electrophoresis compared to other propagation methods. Among the elements analyzed by the pXRF equipment, phosphorous, sulphur, and potassium were prominent, indicating a direct relationship with adventitious rooting in mini cuttings. Based on the tests to investigate possible indications of tissue rejuvenation and/or reinvigoration in E. microcorys, seminal propagation and micropropagation through indirect organogenesis had the strongest relationship with adventitious rooting.
References
Atala LR, Faria JCT, Molinar LV, Avelar MLM, Brondani GE (2022) Reduction of bacterial manifestation in the in vitro cultivation of Eucalyptus microcorys F Muell. Vegetos. https://doi.org/10.1007/s42535-022-00360-z
Aumond ML Jr, Araujo AT Jr, Oliveira Junkes CF, Almeida MR, Matsuura HN, Costa F, Fett-Neto AG (2017) Events associated with early age-related decline in adventitious rooting competence of Eucalyptus globulus Labill. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01734
Azevedo GTDOS, Souza AMD, Azevedo GBD, Teodoro PE, Teodoro LPR, Sousa JRLD (2022) Time of permanence and rooting quality of minicuttings of eucalypt clones. South for 84(1):44–51. https://doi.org/10.2989/20702620.2021.2017761
Baccarin FJB, Brondani GE, Almeida LV, Vieira IG, Oliveira LS, Almeida M (2015) Vegetative rescue and cloning of Eucalyptus benthamii selected adult trees. New for 46(4):465–483. https://doi.org/10.1007/s11056-015-9472-x
Barbosa J, Carvalho MAD, Oliveira LSD, Konzen ER, Campos WF, Brondani GE (2016) Propagation of Khaya anthotheca: interspecific grafting with Swietenia macrophylla and air layering. Cerne 22:475–484. https://doi.org/10.1590/01047760201622042232
Baron D, Amaro ACE, Pina A, Ferreira G (2019) An overview of grafting re-establishment in woody fruit species. Sci Hortic 243:84–91. https://doi.org/10.1016/j.scienta.2018.08.012
Bouranis DL, Malagoli M, Avice JC, Bloem E (2020) Advances in plant sulfur research. Plants 9(2):256. https://doi.org/10.3390/plants9020256
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brondani GE, Baccarin FJB, Gonçalves AN, Almeida MD (2014) Nutritional content in Eucalyptus benthamii mini-stump leaves. Acta Sci Agron 36:465–474. https://doi.org/10.4025/actasciagron.v36i4.16827
Celletti S, Pii Y, Valentinuzzi F, Tiziani R, Fontanella MC, Beone GM, Mimmo T, Cesco S, Astolfi S (2020) Physiological responses to Fe deficiency in split-root tomato plants: possible roles of auxin and ethylene? Agronomy 10(7):1000. https://doi.org/10.3390/agronomy10071000
Clausing S, Pena R, Song B, Müller K, Mayer-Gruner P, Marhan S, Polle A (2021) Carbohydrate depletion in roots impedes phosphorus nutrition in young forest trees. New Phytol 229(5):2611–2624. https://doi.org/10.1111/nph.17058
Cunha ACMM, de Paiva HN, Xavier A, Otoni WC (2009a) The role of mineral nutrition on adventitious rooting in woody plant species. Pesq Flor Bras 58:35–35. https://doi.org/10.4336/2009.pfb.58.35
Cunha ACMCMD, Paiva HND, Barros NFD, Leite HG, Leite FP (2009b) Relationship between the nutritional status of ministumps to the rooting of eucalypt minicuttings. Rev Bras Cienc Solo 33:591–599. https://doi.org/10.1590/S0100-06832009000300012
Cunha ACMCMD, Paiva HND, Leite HG, Barros NFD, Leite FP (2009c) Influence of the nutritional status of ministumps on the rooting of eucalypt minicuttings. Rev Arvore 33:607–615. https://doi.org/10.1590/S0100-67622009000400003
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174(2):332–341. https://doi.org/10.1111/j.1469-8137.2007.01995.x
Faria JCT, Terra JAP, Molinari LV, Delarmelina WM, Ribeiro-Kumara C, Sena Neto AR, Carvalho D, Brondani GE (2021) Use of polylactic acid microvessel to obtain microplantlets of Eucalyptus microcorys through indirect organogenesis. 3 Biotech 11(8):1
Faria JCT, Ribeiro-Kumara C, Costa RSR, Nieri EM, Carvalho D, Pinto JEBP, Neto ARS, Brondani GE (2022) Use of biodegradable polyester-based microvessels for micropropagation of mature Eucalyptus microcorys. NZ J for Sci 52:10. https://doi.org/10.33494/nzjfs522022x139x
García-Limones C, Hervás A, Navas-Cortés JA, Jiménez-Dı́az RM, Tena M (2002) Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol Mol Plant Pathol 61(6):325–337. https://doi.org/10.1006/pmpp.2003.0445
Gong WT, Niu LJ, Wang CL, Wei LJ, Pan Y, Liao WB (2022) Hydrogen peroxide is involved in salicylic acid-induced adventitious rooting in cucumber under cadmium stress. J Plant Biol 65(1):43–52. https://doi.org/10.1007/s12374-021-09332-3
Gonin M, Bergougnoux V, Nguyen TD, Gantet P, Champion A (2019) What makes adventitious roots? Plants 8(7):240. https://doi.org/10.3390/plants8070240
Han H, Sun XM, Xie YH, Feng J, Zhang SG (2014) Transcriptome and proteome profiling of adventitious root development in hybrid larch (Larix kaempferi × Larix olgensis). BMC Plant Biol 14(1):1–13. https://doi.org/10.1186/s12870-014-0305-4
Hartmann H, Kester D, Davies F, Geneve R (2017) Hartmann & kester’s plant propagation: principles and practices. Ed. Pearson, 9th edition, 1024
Higashi EN, Silveira RLVA, Gonçalves AN (2000) Eucalyptus vegetative propagation: principles and its evolution in Brazil. Circular Técnica IPEF 192:1–11
Higashi EN, Silveira RLVA, Gonçalves AN (2004) Nutritional monitoring and fertilization in clonal macro, mini and microgardens. In: gonçalves JLM, Benedeti V (Eds.) Forest Nutrition and Fertilization. Ed. IPEF chapter 8:195–221.
IPEF (1984) Provenances of Eucalyptus spp. introduced in Brazil by different entities. Boletim Inf IPEF Piracicaba 10(29):159
Kang WEI, Wang LY, Li RUAN, Zhang CC, Wu LY, Li HL, Cheng H (2018) Endogenous nitric oxide and hydrogen peroxide detection in indole-3-butyric acid-induced adventitious root formation in Camellia sinensis. J Integr Agric 17(10):2273–2280. https://doi.org/10.1016/S2095-3119(18)62059-3
Koch J, Chakraborty S, Li B, Kucera JM, Van Deventer P, Daniell A, Faul C, Man T, Pearson D, Duda B, Weindorf CA, Weindorf DC (2017) Proximal sensor analysis of mine tailings in South Africa: an exploratory study. J Geochem Explor 181:45–57. https://doi.org/10.1016/j.gexplo.2017.06.020
Lima MDR, Barros Junior UDO, Batista BL, Lobato AKDS (2018) Brassinosteroids mitigate iron deficiency improving nutritional status and photochemical efficiency in Eucalyptus urophylla plants. Trees 32(6):1681–1694. https://doi.org/10.1007/s00468-018-1743-7
Lloyd C, McCown B (1980) Commercially-feasible micropropagation of mountain laurel Kalmia latifolia, by use of shoot-tip culture. Int Plant Prop Soc Proc 30:421–427
Marschner P (2012) Marschner’s Mineral nutrition of higher plants. Elsevier/Academic Press
Mateus NS, de Oliveira Ferreira EV, Junior JCA, Domec JC, Jordan-Meille L, de Moraes Gonçalves JL, Lavres J (2019) The ideal percentage of K substitution by Na in Eucalyptus seedlings: evidences from leaf carbon isotopic composition, leaf gas exchanges and plant growth. Plant Physiol Biochem 137:102–112. https://doi.org/10.1016/j.plaphy.2019.02.006
McGladdery C, Weindorf DC, Chakraborty S, Li B, Paulette L, Podar D, Pearson D, Kusi NYO, Duda B (2018) Elemental assessment of vegetation via portable X-ray fluorescence (PXRF) spectrometry. J Environ Manage 210:210–225. https://doi.org/10.1016/j.jenvman.2018.01.003
Mendonça EG, Batista TR, Stein VC, Balieiro FP, Abreu JR, Pires MF, Souza PA, Paiva LV (2020) In vitro serial subculture to improve rooting of Eucalyptus urophylla. New for 51(5):801–816. https://doi.org/10.1007/s11056-019-09761-6
Mercado IV, Narváez I, Ríos EP, Litz RE, Muñoz AB, Alfaro FP (2021) Reinvigoration/rejuvenation induced through micrografting of tree species: signaling through graft union. Plants 10(6):1197. https://doi.org/10.3390/plants10061197
Müller C, Hodecker BER, Merchant A, Barros NFD (2017) Nutritional efficiency of Eucalyptus clones under water stress. Rev Bras Cienc Solo 41:1–17. https://doi.org/10.1590/18069657rbcs20160528
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Narayan OP, Kumar P, Yadav B, Dua M, Johri AK (2022) Sulfur nutrition and its role in plant growth and development. Plant SIgnaling Behav. https://doi.org/10.1080/15592324.2022.2030082
Oliveira FN, Fortes GA, Paula JR, Ferri PH, Santos SC (2014) Seasonal influence on the essential oil of Eucalyptus microcorys. Nat Prod Commun 9(4):575–580. https://doi.org/10.1177/1934578X1400900439
Oliveira LS, Brondani GE, Batagin-Piotto KD, Calsavara R, Gonçalves AN, de Almeida M (2015) Micropropagation of Eucalyptus cloeziana mature trees. Aust for 78(4):219–231. https://doi.org/10.1080/00049158.2015.1073211
Pereira FB, Peres FSB (2016) Nutrition and adventitious rooting in woody plants. Pesq Flor Bras 36(87):319–326. https://doi.org/10.4336/2016.pfb.36.87.1146
Pina A, Cookson SJ, Calatayud A, Trinchera A, Errea P (2017) Physiological and molecular mechanisms underlying graft compatibility. In: Colla G, Pérez-Alfocea F, Schwarz D (eds) Vegetable Grafting: principles and Practices Wallingford. CABI
Prado DZ, Dionizio RC, Vianello F, Magro M, Lima GPP (2014) Can hydrogen peroxide and quercetin improve production of Eucalyptus grandis × Eucalyptus urophylla? Afr J Biotechnol. https://doi.org/10.5897/AJB2014.13792
Rodríguez AGM, Bucio JL, Sánchez GR, Herrera LFR, Abud YC, Trujillo MM (2020) Chromium differentially affects hydrogen peroxide distribution in primary and adventitious roots of Arabidopsis thaliana L. Phyton 89(1):35
Santos Junior CF, Costa MD, Rech TD, Boff P, Boff MIC (2021) Use of micropropagation in the vegetative rescue of adult trees of Cedrela odorata L. Rev Bras Cienc Agrar 16(4):1–7
Silva MKF, Siqueira DP, Carvalho GCMW, Silva RD, Silva RMR, Barroso DG (2022) Hydrogen peroxide enhanced indole-3-butyric acid effects on Cordia trichotoma adventitious rooting. Rhizosphere 22:100533. https://doi.org/10.1016/j.rhisph.2022.100533
Soares TP, Pozza EA, Pozza AA, Mafia RG, Ferreira MA (2018) Calcium and potassium imbalance favours leaf blight and defoliation caused by Calonectria pteridis in Eucalyptus plants. Forests 9(12):782. https://doi.org/10.3390/f9120782
Song Y, Ling N, Ma JH, Wang JC, Zhu C, Raza W, Shen YF, Huang QW, Shen QR (2016) Grafting resulted in a distinct proteomic profile of watermelon root exudates relative to the un-grafted watermelon and the rootstock plant. J Plant Growth Regul 35(3):778–791. https://doi.org/10.1007/s00344-016-9582-5
Sustr M, Soukup A, Tylova E (2019) Potassium in root growth and development. Plants 8(10):435. https://doi.org/10.3390/plants8100435
Takáč T, Obert B, Rolčík J, Šamaj J (2016) Improvement of adventitious root formation in flax using hydrogen peroxide. New Biotechnol 33(5):728–734. https://doi.org/10.1016/j.nbt.2016.02.008
Teixeira GC, Konzen ER, Faria JCT, Golçalves DS, Carvalho D, Brondani GE (2020) Genetic diversity analysis of two Eucalyptus species using ISSR markers. Cienc Florest 30(1):270–278. https://doi.org/10.5902/1980509832804
Towett EK, Shepherd KD, Lee Drake B (2016) Plant elemental composition and portable X-ray fluorescence (pXRF) spectroscopy: quantification under different analytical parameters. Xray Spectrom 45(2):117–124. https://doi.org/10.1002/xrs.2678
Vatansever R, Filiz E, Ozyigit II (2016) In silico analysis of Mn transporters (NRAMP1) in various plant species. Mol Biol Rep 43(3):151–163. https://doi.org/10.1007/s11033-016-3950-x
Vilasboa J, Costa CT, Ransan LG, Mariath JEDA, Fett-Neto AG (2021) Microcutting redox profile and anatomy in Eucalyptus spp. with distinct adventitious rooting competence. Front Plant Sci 11:620832. https://doi.org/10.3389/fpls.2020.620832
Wang LP, Li GJ, Wu XH, Xu P (2016) Comparative proteomic analyses provide novel insights into the effects of grafting wound and hetero-grafting per se on bottle gourd. Sci Hortic 200:1–6. https://doi.org/10.1016/j.scienta.2015.12.056
Wei K, Ruan L, Wang LY, Cheng H (2019) Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int J Mol Sci 20(19):4817. https://doi.org/10.3390/ijms20194817
Weindorf DC, Chakraborty S (2020) Portable X-ray fluorescence spectrometry analysis of soils. Soil Sci Soc Am J 84(5):1384–1392. https://doi.org/10.1002/saj2.20151
Weindorf DC, Bakr N, Zhu YD (2014) Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Adv Agron 128:1–45. https://doi.org/10.1016/B978-0-12-802139-2.00001-9
Wendling I, Xavier A (2005) Influência da miniestaquia seriada no vigor radicular de clones de Eucalyptus grandis. Rev Arvore 29:681–689
Wendling I, Trueman SJ, Xavier A (2014) Maturation and related aspects in clonal forestry—Part I: concepts, regulation and consequences of phase change. New for 45(4):449–471. https://doi.org/10.1007/s11056-014-9421-0
Xavier A, Wendling L, Silva RL (2021) Silvicultura clonal: princípios e técnicas. Ed. UFV, 3th edition, Viçosa, pp. 257.
Xu DB, Yuan HW, Tong YF, Zhao L, Qiu LL, Guo WB, Shen CJ, Liu HJ, Yan DL, Zheng BS (2017) Comparative proteomic analysis of the graft unions in hickory (Carya cathayensis) provides insights into response mechanisms to grafting process. Front Plant Sci 8:676. https://doi.org/10.3389/fpls.2017.00676
Zhang S, Zhao Z, Zhang LL, Zhou QY (2015) Comparative proteomic analysis of tetraploid black locust (Robinia pseudoacacia L.) cuttings in different phases of adventitious root development. Trees 29(2):367–384. https://doi.org/10.1007/s00468-014-1116-9
Zhou SB, Weindorf DC, Cheng QM, Yang BY, Yuan ZX, Chakraborty S (2020) Elemental assessment of vegetation via portable X-ray fluorescence: sample preparation and methodological considerations. Spectrochim Acta Part B 174:105999. https://doi.org/10.1016/j.sab.2020.105999
Funding
This study was supported by the Coordination for the Improvement of Higher Education Personnel–Brazil (CAPES), the National Council for Scientific and Technological Development (CNPq), and the Research Support Foundation of the State of Minas (FAPEMIG).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding : This study was supported by the Coordination for the Improvement of Higher Education Personnel–Brazil (CAPES), the National Council for Scientific and Technological Development (CNPq), and the Research Support Foundation of the State of Minas (FAPEMIG).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faria, J.C.T., Ribeiro-Kumara, C., Delarmelina, W.M. et al. Evaluation of total protein, peroxidase, and nutrients measured by pXRF for the determination of tissue rejuvenation/reinvigoration of Eucalyptus microcorys. J. For. Res. 34, 1563–1576 (2023). https://doi.org/10.1007/s11676-022-01585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01585-z