Abstract
The rooting capacity of Pinus massoniana is poor, especially for mature trees, and has prevented the development of clonal forestry for P. massoniana. In this study, we varied explant types, subculture times and exogenous hormones for plantlet regeneration and assessed shoots for rooting rate and root number for P. massoniana. Following five repetitive grafts, new shoots from grafts used as explant sources were rejuvenated as observed from juvenile shoot morphology and anatomy, leading to greatly enhanced plant regeneration in comparison to that of mature materials from 26-year-old P. massoniana trees. The rooting capacity of subcultured shoots increased with successive subcultures, reaching a peak at 20 subcultures with 35–40 days per subculture. However, rooting performance was significantly reduced after 30 subcultures. The addition of naphthaleneacetic acid (NAA) plus indoleacetic acid in the medium improved the root number, but the combination of exogenous NAA with paclobutrazol (PBZ) increased rooting rate and root number. We thus greatly improved the rooting capacity of mature P. massoniana trees by optimizing explant types (rejuvenated), subculture times (20 subcultures, 35–40 days per subculture) and addition of NAA + PBZ to the rooting medium. The conditions can be used for efficient plantlet regeneration of P. massoniana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pinus massoniana, cultivated for its timber and natural resin (Zhu et al. 2010), has an important role in afforestation in southern China. Currently, plantations have been started mainly from seeds, which leads to low-productivity forest stands, severely limiting the commercialization of this important tree (Wang and Yao 2019). For promoting the development of the P. massoniana industry, clonal forestry is very important, as shown by the rapid growth of Eucalyptus plantations and the forestry economy after commercialized application of selected genotypes (Yang et al. 1995).
For achieving the desired properties of trees, however, clones can only be selected when the trees have reached maturity (Basheer-Salimia 2007). For most mature trees, plantlet regeneration is problematic with respect to adventitious rooting. Pinus massoniana is recalcitrant to rooting, and its rooting capacity is greatly decreased with physiological aging (Zhu et al. 2010; Yao and Wang 2016; Wang and Yao 2019). To successfully propagate selected genotypes in vitro, we thus need to improve the rooting capacity of mature P. massoniana trees.
Adventitious root (AR) formation can be significantly improved following rejuvenation of mature plants (Bonga 1987; Day et al. 2002). Mature materials can be rejuvenated by various methods such as pruning, etiolation, successive subculturing and repeated grafting (Wendling et al. 2014). Phenotypes and organizational structures of mature and juvenile trees can easily be distinguished (Day et al. 2002; Poethig 2003). The degree of rejuvenation can be determined by evaluating cellular and subcellular anatomical structures; for example, abundant meristematic cells, thin nuclear chromatin, small nucleoli and paucity of cell organelles are juvenile characteristics of trees (Bonga 1987; Greenwood et al. 1989; Day et al. 2002).
We previously regenerated plantlets of mature trees with a rooting rate of 65.3% in P. massoniana (Wang and Yao 2017), but this micropropagation protocol is not appropriate for commercial application. In addition, in recent in vitro culturing, the efficiency of plantlet regeneration was very poor, resulting from a decreased rooting capacity after long-term subculture, which greatly prevents the development of clonal industrialization in P. massoniana. It is clear that the number of subcultures is closely related to physiological changes in the subcultured shoots, such as the vigor and growth of shoots, leading to variations in AR development (Shi et al. 2007). Successive subculture contributes to the rejuvenation of in vitro cultured materials, while in general, the capacity of regeneration in vitro declines after long-term subcultures of woody plants (Su 2000).
IAA is important for regulating adventitious rooting (Huang et al. 2007) and has been widely applied to synchronize AR formation and improve the root quality of phenotypes with poor rooting capacity (Geiss et al. 2009). The role of GA is similar to that of IAA in regulating AR formation in general, promoting cell differentiation and elongation (Yamaguchi 2008). However, some studies show that GA might inhibit AR formation (Eriksson et al. 2000; Lombardi-Crestana et al. 2012). Mauriat et al. (2011) suggested that the inhibitory effects of GA on AR formation are due to altered IAA transport. Consequently, an inhibitor of GA biosynthesis, paclobutrazol (PBZ), is extensively used to promote root development (Watson 1996, 2004; Salari et al. 2017; Kamran et al. 2018).
Most of the 116 varieties of the world’s conifers belonging to the genus Pinus (Huang and Wei 1994) are recalcitrant to rooting. Here we used 26-year-old P. massoniana trees to test the effects of explant types (mature or rejuvenated), subculture times and exogenous hormones on rooting capacities to develop an efficient protocol that optimizes in vitro rooting during plantlet regeneration of mature P. massoniana trees.
Materials and methods
Plant materials
Mature mother trees used in the present study were selected from 26-year-old superior stands of P. massoniana ‘Tongmiansong’ (TM provenance), which is widely distributed throughout southern China. According to the method of Wang and Yao (2017), tender shoots from mother trees were grafted onto 1-year-old in vitro cultured seedlings of P. massoniana. The tops of the grafted plants were pruned to produce axillary shoots when they reached approximately 0.5 m high. New shoots from the pruned grafts were used as mature materials.
For rejuvenating the materials, new shoots from mother trees were first grafted to rootstock. According to the method described above, when the grafted plants reached 0.5 m, the tops were pruned to produce axillary shoots, which were then used for the next grafting. After five such graftings, new tender shoots with fasciculate needles from the graftlings were used as rejuvenated materials.
Observations of morphology and anatomy
To examine variations in organizational structure after rejuvenation, 10 basal segments, approximately 1 cm long, were excised from new shoots of the mature and rejuvenated materials. After a wash with sterilized water, all shoots were fixed, embedded, stained, rinsed and mounted using the method of Wang and Yao (2019). Images of changes in the anatomy and ultrastructure of shoots were captured using a BX41-12P02 light microscope (Olympus, Tokyo, Japan) or H-600 transmission election microscope (Hitachi, Tokyo, Japan). For each sample, three replicates and 10 microscopic fields per replicate were assessed.
Plantlet regeneration
According to the in vitro culture conditions described by Yao and Wang (2016), nodal segments taken from new shoots of mature and rejuvenated materials were sterilized and placed on modified Murashige–Skoog (MS) (Murashige and Skoog 1962) medium (MMS medium, Yao and Wang 2016) hormones added to the medium to induce initial bud were the same as those we previously used (Wang and Yao 2019). Once axillary buds sprouted and grew to 2–3 cm in length, the shoots were excised from nodal segments and subcultured every 35–40 days on MMS medium containing hormones at a third of the amount applied for the initial bud induction. Following four subcultures, in vitro cultured shoots ≧ 20 mm were transferred into half-strength MMS medium containing 1.2 μM naphthaleneacetic acid (NAA). After rooting induction for 60 days, the rooted shoots were acclimated in the nursery as described previously (Wang and Yao 2017). Surviving explants with initial buds were counted to determine the induction rate 75 days after initial bud induction. The number of axillary buds induced per subcultured shoot was recorded as the proliferation coefficient from each subculture. The number of rooted shoots was used to calculate the rooting rate. The survival rate was expressed as the percentage of surviving plantlets among total plantlets 3 months after transplanting. In this experiment, 100 new shoots (10 explants × 5 replicates × 2 explant types) and 120 subcultured shoots (12 subcultured shoots × 5 replicates × 2 explant types) were tested.
Rooting capacity analyses
Thirty subcultures were performed with a subculture cycle of 35–40 days per subculture. To clarify the impacts of subculture generations on rooting, subcultured shoots originating from rejuvenated materials after the 1st, 5th, 10th, 20th and 30th subcultures were sequentially collected for rooting induction. The rooting medium was the same as that described above. Here, the rooting rate and root number were individually determined. For the root number, the number of roots ≥ 1 cm per rooted shoot was recorded. A total of 300 subcultured shoots (12 subcultured shoots × 5 replicates × 5 subcultures × 1 explant type) were sequentially collected for rooting induction.
Determination of exogenous hormone effects
Toward enhancing rhizogenesis of long-term subcultured shoots with poor rooting capacity, subcultured shoots from rejuvenated materials after subculturing 30 times were cultured in rooting medium containing 1.2 μM NAA and 2 μM IAA or 2 μM paclobutrazol (PBZ), and medium including 1.2 μM NAA was used as the control treatment. Rooting rate and root number were determined as before. A total of 180 subcultured shoots (12 subcultured shoots × 5 replicates × 3 hormones treatments × 1 subculture × 1 explant type) were sampled in this experiment.
Data analyses
The statistical analyses used were a factorial ANOVA (with explant types, subculture times or hormone treatments as factors), t test (significant difference between explant types) and Duncan’s test (significant difference among subculture times or exogenous hormones treatments). Variance analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
Results
Changes in histology and anatomy
Morphological, histological and subcellular differences between rejuvenated and mature shoots are shown in Fig. 1. Shoots with low lignification were observed following five repeated graftings, and only the top needles of the buds were clustered on these shoots (Fig. 1a, b). Histological changes barely differed between mature and repeated grafting shoots, but the number of sclerenchyma cells around the cambial tissues was reduced in the repeatedly grafted shoots (Fig. 1c, d). At the subcellular level, the size of the nucleus and the degree of nuclear chromatin condensation significantly differed between mature and rejuvenated materials (Fig. 1e, f). In mature shoots, chromatin condensation and a large nucleus were clearly evident. Furthermore, an endoplasmic reticulum around the nucleus was also found (Fig. 1e). After rejuvenation treatment, the nucleus became smaller (Fig. 1f). Generally, plastids were easily visible in the shoots regardless of the material source (Fig. 1e, f).
Morphological and anatomical details of shoots from mature and rejuvenated materials in Pinus massoniana. a and b Morphology of shoots tested in this study: mature (a), rejuvenated (b). c–f Histological and subcellular details of shoots: mature (c, e), rejuvenated (d, f). Cy cytoplasm, ER endoplasmic reticulum, Nu nucleus, NC nuclear chromatin, P plastid, V vacuole. Arrows: blue, resin canals; yellow, sclerenchyma; red, meristematic cells. Scale bars: 2 cm (a, b), 200 µm (c, d), 0.5 µm (e, f)
Plantlet regeneration performance
In terms of observations of histological and anatomical changes, new shoots of 26-year-old P. massoniana trees were rejuvenated through five successive graftings. In comparison to explants from mature materials, the induction rate, proliferation coefficient, rooting rate and survival rate of transplanted plants were significantly enhanced, reaching 82.2%, 5.9 plant−1, 90.8% and 91.2%, respectively (Fig. 2). In terms of improving the rooting performance by rejuvenation, the rooting rate of subcultured shoots from the rejuvenated explants was 8.7 times higher than that from mature explants (Fig. 2).
Effects of explant rejuvenation on in vitro plantlet regeneration of mature trees of Pinus massoniana. Lowercase letters indicate differences in induction rate, proliferation coefficient, rooting rate or survival rate between the two explant sources (P < 0.05; t test). Bars in the figure indicate standard errors (SE)
Variations in rooting capacity
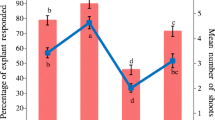
We developed micropropagation systems for 257 genotypes with the rejuvenated explants by repetitive grafting in P. massoniana (Fig. 3a–d). The selected genotypes were further proliferated for practical use through successive subcultures over nearly 4 years, which caused declines in shoot vigor and rooting capacity (Fig. 3f, h). From Fig. 4, it was clear that rooting capacity increased with the number of subcultures when that number was less than 20, and the rooting rate and root number were much higher at the 10th and 20th subcultures. However, both of these rooting variables significantly decreased after 30 subcultures (Fig. 4).
Establishment of the in vitro regeneration system for superior mature trees of Pinus massoniana. a Grafts using tender shoots of selected mature trees. b New shoots of grafts as explants. c Induction of initial buds from nodal segments. d, In vitro sterile preservation of different genotypes. e Vigorous subcultured shoots. f Less-vigorous subcultured shoots after long-term subculturing. g Typical rooted shoots after induction with 1.2 µM NAA. h Rooted shoots after long-term subculturing with 1.2 µM NAA. i Rooted shoots after long-term subculturing with 1.2 µM NAA + 2 µM PAC. Scale bars: a 2 cm; b, c, e, f, h and i 1 cm; d 10 cm; g 5 cm
Variations in rooting capacity of subcultured shoots during long-term subcultures from rejuvenated materials of 26-year-old trees of Pinus massoniana. Lowercase letters indicate differences in the rooting rate or root number among subculture times (P < 0.05; Duncan’s test). Bars in the figure indicate standard errors (SE)
Effects of exogenous hormones
As shown in Fig. 5, the differences in rooting rate and root number of long-term subcultured shoots were significant among the three exogenous hormone treatments. Compared to the control (NAA), root number was enhanced in both the NAA + IAA and the NAA + PBZ treatments, while rooting rate increased only in the NAA + PBZ treatment. There was no significant difference in rooting rate between the NAA and NAA + IAA treatments.
Effects of exogenous hormones on rooting capacity of long-term (40 subcultures, 35–40 days for each subculture)-subcultured shoots from rejuvenated explants in 26-year-old Pinus massoniana trees. Lowercase letters indicate differences in the rooting rate or root number among hormone treatments (P < 0.05; Duncan’s test). Bars in the figure indicate standard errors (SE)
Discussion
Usually, juvenile trees grow vigorously and hence are capable of in vitro rooting, while adventitious rooting capacity is gradually reduced when trees reach maturity (Pijut et al. 2011). Numerous studies have reported that the formation of adventitious roots improved after certain rejuvenation treatments in adult trees. In mature Castanea sativa trees, the rooting effect was inconsistent and varied in the range of 10–70%; however, its rooting ability increased remarkably after grafting four times (Giovannelli and Giannini 2000). For walnut propagation in vitro, the rooting rate was enhanced with a dark pretreatment (Vahdati et al. 2004). In the present study, our findings showed that the rooting capacity of mature P. massoniana trees was improved through repetitive grafting. The induction rate, proliferation coefficient, rooting rate and survival percentage after transplanting of shoots were significantly higher than those of mature materials (Fig. 3). This finding suggested that successive grafting could be used as an effective method to rejuvenate mature trees in Pinus species.
In our experiments, the anatomical structure changed during the rejuvenation. Clearly, the size of the nucleolus diminished, and discontinuously distributed sclerenchyma cells were easily observed (Fig. 2d–i). Physiological and biochemical metabolism are closely related to the nucleolus in plants. In juvenile plants, the nucleolus is much smaller (Bonga 1987; Day et al. 2002). Previous studies have reported that discontinuous sclerenchyma tissue was beneficial for the induction of adventitious roots (Maynard et al. 1996). Our results suggest that the continuity of sclerenchyma tissues was possibly linked with tree physiological age, resulting in reduced rooting capacity in adult trees.
Subculture time influences plantlet regeneration (Su 2000; Shi et al. 2007). For P. massoniana, successive subcultures also led to variations in rooting capacity. After 20 subcultures, the rooting rate and root number of shoots were significantly higher than those of shoots subcultured 1–5 times. In view of the gradually increased rooting capacity with subculture times during 20 successive subcultures, our findings indicated that the protocol to enhance rhizogenesis ability could be more efficient in mature P. massoniana trees when repetitive graftings were combined with successive subcultures. However, the observed decrease in rooting capacity after 30 subcultures shows the inhibitory impact of long-term subculture on adventitious rooting in P. massoniana. Considering that the optimal number of subculture cycles varies with tree species (Su 2000; Shi et al. 2007), it is necessary to adjust and optimize the subculture time to obtain stable micropropagation performance in P. massoniana. In this study, approximately 2 years of successive subculture (20 subcultures, 35–40 days per subculture) seems to be the optimum for adventitious root development in subcultured shoots of P. massoniana.
Hormones affect the growth and development of plants. NAA, an auxin analogue, is extensively applied in rooting medium in P. massoniana (Li et al. 2009; Yang et al. 2011). In our previous research, we confirmed that supplementation with 1.2 μM NAA in the medium was effective for rooting induction in P. massoniana (Wang and Yao 2019) but was no longer effective with up to 30 subcultures, as shown in Fig. 3h. Auxin (IAA) plays an important role in regulating adventitious root formation, and the action of GA affecting rooting in vitro due to hormonal crosstalk between IAA and GA has been clarified in plants (Fu and Harberd 2003). In recent studies, the negative effects of GA on adventitious root development have been highlighted (Mauriat et al. 2014). Hence, exogenous NAA was combined with IAA or PBZ, an inhibitor of GA synthesis, and added to the rooting medium in the present study to improve plantlet regeneration in long-term subcultured shoots with poor rooting ability. Compared with NAA treatment alone, the addition of IAA increased the root number, suggesting that the role of IAA in regulating rhizogenesis is mainly related to root number rather than the rooting rate. We speculate that IAA, a promoter of adventitious roots, could positively increase root formation (root number) but not effectively improve root growth (rooting rate). However, supplementation with PBZ increased the rooting rate and root number in comparison to the NAA treatment, suggesting that GA negatively affects root growth and formation. To further demonstrate the effect of GA on rooting, investigations on the relationship between GA and rooting capacity are needed.
In conclusion, the plantlet regeneration of mature trees was improved in P. massoniana following five graftings. Successive subculture was beneficial for increasing the rooting capacity during approximately 2 years of subculturing, while rooting performance declined after long-term subculturing (> 3 years). For long-term-subcultured shoots, exogenous IAA added to the medium promoted root formation, and the application of PBZ in the rooting medium caused increases in root growth and root formation. The present findings are a great advancement for the efficient micropropagation of selected genotypes in P. massoniana. Regarding the poor rooting capacity in Pinus species, more positive responses of plant regeneration to exogenous PBZ in culture medium can be expected.
References
Basheer-Salimia R (2007) Juvenility, maturity, and rejuvenation in woody plants. Hebron Univ Res J 3:17–43
Bonga JC (1987) Clonal propagation of mature trees: problems and possible solutions. In: Bonga JM, Durzan DJ (eds) Cell and tissue culture in forestry, general principles and biotechnology. Martinus Nijhoff, Dordrecht, pp 249–271
Day ME, Greenwood MS, Diaz-Sala C (2002) Age-and size-related trends in woody plant shoot development: regulatory pathways and evidence for genetic control. Tree Physiol 22:507–513
Eriksson ME, Israelsson M, Olsson O, Moritz T (2000) Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotech 18:784–788
Fu XD, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743
Geiss G, Gutierrez L, Bellini C (2009) Adventitious root formation: new insights and perspectives. In: Beekman T (ed) Root development. Wiley-Blackwell, Oxford, p 376
Giovannelli A, Giannini R (2000) Reinvigoration of mature chestnut (Castaneasativa) by repeated graftings and micropropagation. Tree Physiol 20:1243–1248
Greenwood MS, Hopper CA, Hutchison KW (1989) Maturation in Larch: 1. Effect of age on shoot growth, foliar characteristics and DNA methylation. Plant Physiol 90:406–412
Huang JQ, Wei ZM (1994) Tissue and protoplast culture of Pinus species. Chin Bull Bot 11(1):34–42
Huang Y, Ji KS, Zhai JR (2007) Relationship between rooting ability and endogenous phytohormone changes in successive continuous generation cuttings of Buxussinica var. parvifolia, an endangered woody species in China. Forest Stud China 9:189–197
Kamran M, Wennan S, Ahmad I, Meng XP, Cui WW, Zhang XD, Mou SW, Kha A, Han QF, Liu TN (2018) Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci Rep. https://doi.org/10.1038/s41598-018-23166-z
Li XY, Lv CQ, Huang BL, Wu QM, Zhang MH (2009) Adventitious roots induction of Pinusmassoniana shoots in test tubes and anatomical observation. J Northwest For Univ 24(3):80–84
Lombardi-Crestana S, da Silva Azevedo M, e Silva GFF, Pino LE, Appezzato-da-Gloria B (2012) The tomato (Solanumlycopersicumcv. Micro-Tom) natural genetic variation Rg1 and the DELLA mutant procera control the competence necessary to form adventitious roots and shoots. J Exp Bot 63:5689–5703
Mauriat M, Sandberg LG, Moritz T (2011) Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J 67(5):805–816
Mauriat M, Petterle A, Bellini C, Moritz T (2014) Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J 78:372–384
Maynard BK, Bassuk NL, Maynard BK (1996) Effects of stock plant etiolation, shading, banding, and shoot development on histology and cutting propagation of Carpinusbetulus L, fastigiata. J Am Soc Hortic Sci 121:853–860
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pijut PM, Woeste KE, Michler CH (2011) Promotion of adventitious root formation of difficult-to-root hardwood tree species. Hortic Rev 38:213–251
Poethig RS (2003) Phase change and the regulation of developmental timing in plants. Science 301:334–336
Salari H, Baninasab B, Akbari M, Rohani AM (2017) Effect of paclobutrazol on adventitious root formation of IBA-treated cuttings of ‘Zard’ and ‘Dakal’ Olive (Oleaeuropaea L.) cultivars. Asian J Appl Sci 5(4):692–699
Shi XX, Du GQ, Wang C, Ma BK, Ge YN (2007) Effects of subculture times on organogenesis characteristics of apple in vitro shoot explants. Acta Hortic Sin 34(3):561–564
Su XC (2000) Study on the differences of the seedling of different generations from successive tissue culture of Chinese fir clone. J Fujian Coll For 20(4):353–356
Vahdati K, Leslie C, Zamani Z, McGranahan G (2004) Rooting and acclimatization of in vitro-grown shoots from mature trees of three Persian walnut cultivars. HortScience 39:324–327
Wang Y, Yao RL (2017) Plantlet regeneration of adult Pinusmassoniana Lamb. trees using explants collected in March and thidiazuron in culture medium. J For Res 28:1169–1175
Wang Y, Yao RL (2019) Increased endogenous indole-3-acetic acid:abscisic acid ratio is a reliable marker of Pinusmassoniana rejuvenation. Biotech Histochem. https://doi.org/10.1080/10520295.2019.1608468
Watson GW (1996) Tree root system enhancement with paclobutrazol. J Arbor 22:211–217
Watson GW (2004) Effect of transplanting and paclobutrazol on root growth of ‘green column’ black maple and ‘Summit’ green ash. J Environ Hortic 22:209–212
Wendling I, Trueman SJ, Xavier A (2014) Maturation and related aspects in clonal forestry-part II: reinvigoration, rejuvenation and juvenility maintenance. New For 45:473–486
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yang JC, Chung JD, Chen ZZ (1995) Vegetative propagation of adult Eucalyptusgrandis × urophylla and comparison of growth between micropropagated plantlets and rooted cuttings. Plant Cell Rep 15:170–173
Yang MH, Zhang DL, Yang Y, Ding GJ, Li ZH (2011) Micropropagation in immature embryos of Pinusmassoniana in vitro. J Central South Univ For Tech 31(3):90–96
Yao RL, Wang Y (2016) An effective protocol for regenerating mature Pinusmassoniana L. trees by tissue culture. Res J Biotech 11:75–80
Zhu LH, Wu XQ, Qu HY, Ji J, Ye JR (2010) Micropropagation of Pinusmassoniana and mycorrhiza formation in vitro. Plant Cell Tissue Organ 102:121–128
Acknowledgements
We also are grateful for the help of American Journal Experts in polishing the language in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by the Project of Scientific and Technological Plan from the Department of Science and Technology of Guangxi under Grants 2018GXNSFDA281020, AD17195078, 2017GXNSFAA198037 and AA17204087-1, the Natural Science Foundation of China under Grant 31960311 and 31360178, and the Key Program of Guangxi Forestry Bureau under Grant [2016]13 and GL2019KT06.
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Yao, R. Optimization of rhizogenesis for in vitro shoot culture of Pinus massoniana Lamb.. J. For. Res. 32, 203–209 (2021). https://doi.org/10.1007/s11676-019-01076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-01076-8