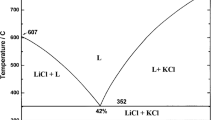
Abstract
The phase diagram of the LiNO3-NaNO3-Ca(NO3)2 ternary system was predicted by using an asymmetric Toop model. The thermogravimetry and Differential Scanning Calorimeter experiments of the predicted eutectic composition were also carried out. The eutectic temperature and composition reported in various literatures are evaluated based on the results determined in this work. The results show that the eutectic temperature and composition reported by Storonkin et al. (Vopr Termodin Geterogen Sist i, 2:128-139, 1973) is more reliable than reported by other literatures. It is indict that wider working temperature window (lower condensation point) in solar thermal power plant can be obtained when the thermal energy storage medium of the titled system is prepared with the eutectic composition determined in this paper.






Similar content being viewed by others
References
R.G. Reddy, Molten Salts: Thermal Energy Storage and Heat Transfer Media, J. Phase Equilib. Diffus., 2011, 32(4), p 269-270
Y. Tian and C.Y. Zhao, A Review of Solar Collectors and Thermal Energy Storage in Solar Thermal Applications, Appl. Energy, 2013, 104, p 538-553
K.H. Solangi, M.R. Islam, R. Saidur, N.A. Rahim, and H. Fayaz, A Review on Global Solar Energy Policy, Renew. Sust. Energy Rev., 2011, 15(4), p 2149-2163
X.L. Wei, Q. Qeng, J. Ding, X.X. Yang, J.P. Yang, and B. Long, Theoretical Study on Thermal Stability of Molten Salt for Solar Thermal Power, Appl. Therm. Eng., 2013, 54(1), p 140-144
Q. Qeng, X.X. Yang, J. Ding, X.L. Wei, and J.P. Yang, Design of New Molten Salt Thermal Energy Storage Materials for Solar Thermal Power Plant, Appl. Energy, 2013, 112, p 682-689
C.Y. Zhao and Z.G. Wu, Thermal Property Characterization of a Low Melting-Temperature Ternary Nitrate Salt Mixture for Thermal Energy Storage Systems, Sol. Energy Mater. Sol. Cells, 2011, 95, p 3341-3346
Y.T. Wu, N. Ren, T. Wang, and C.F. Ma, Experimental Study on Optimized Composition of Mixed Carbonate Salt for Sensible Heat Storage in Solar Thermal Power Plant, Sol. Energy, 2011, 85(9), p 1957-1966
J.C. Gomez, N. Calvet, A.K. Starace, and G.C. Glatzmaier, Ca(NO3)2-NaNO3-KNO3 Molten Salt Mixtures for Direct Thermal Energy Storage Systems in Parabolic Trough Plants, J. Sol. Energy Eng., 2013, 135, p 021016-1-021016-8
D. Kearney, B. Kelly, U. Herrmann, R. Cable, J. Pacheco, R. Mahoney, H. Price, D. Blake, P. Nava, and N. Potrovitza, Engineering Aspects of a Molten Salt Heat Transfer Fluid in a Trough Solar Field, Energy, 2004, 29(5-6), p 861-870
D. Kearney, U. Herrmann, P. Nava, B. Kelly, R. Mahoney, J. Pacheco, R. Cable, N. Potrovitza, D. Blake, and H. Price, Assessment of a Molten Salt Heat Transfer Fluid in a Parabolic Trough Solar Field, J. Sol. Energy Eng., 2003, 125(2), p 170-176
R.G. Reddy, T. Wang, and D. Mantha, Thermodynamic Properties of Potassium Nitrate-Magnesium Nitrate Compound [2KNO3·Mg(NO3)2], Thermochim. Acta, 2012, 531, p 6-11
R.I. Olivares and W. Edwards, LiNO3-NaNO3-KNO3 Salt for Thermal Energy Storage: Thermal Stability Evaluation in Different Atmospheres, Thermochim. Acta, 2013, 560, p 34-42
D. Mantha, T. Wang, and R.G. Reddy, Thermodynamic Modeling of Eutectic Point in the LiNO3-NaNO3-KNO3 Ternary System, J. Phase Equilib. Diffus., 2012, 33(2), p 110-114
T. Wang, D. Mantha, and R.G. Reddy, Novel Low Melting Point Quaternary Eutectic System for Solar Thermal Energy Storage, Appl. Energy, 2013, 102, p 1422-1429
T. Wang, D. Mantha, and R.G. Reddy. High Thermal Energy Storage Density LiNO3-NaNO3-KNO3-KNO2 Quaternary Molten Salt for Parabolic Trough Solar Power Generation, Energy Technol., 2012, p 73-84
A.G. Fernández, S. Ushak, H. Galleguillos, and F.J. Pérez, Thermal Characterisation of an Innovative Quaternary Molten Nitrate Mixture for Energy Storage in CSP Plants, Sol. Energy Mater. Sol. Cells, 2015, 132, p 172-177
H.R. Carveth, Study of a Three-Component System, J. Phys. Chem., 1898, 2(4), p 209-228
R.W. Bradshaw and D.E. Meeker, High-Temperature Stability of Ternary Nitrate Molten Salts for Solar Thermal Energy Systems, Sol. Energy Mater., 1990, 21(1), p 51-60
F. Roget, C. Favotto, and J. Rogez, Study of the KNO3-LiNO3 and KNO3-NaNO3-LiNO3 Eutectics as Phase Change Materials for Thermal Storage in a Low-Temperature Solar Power Plant, Sol. Energy, 2013, 95, p 155-169
A. Lehrman, E. Adler, J. Freidus, and M. Neimand, The Liquidus Curve and Surface of the Systems Lithium and Calcium Nitrates and Calcium, Lithium and Potassium Nitrates, J. Am. Chem. Soc., 1937, 59(1), p 179-181
C.Y. Zhao and Z.G. Wu, Thermal Property Characterization of a Low Melting-Temperature Ternary Nitrate Salt Mixture for Thermal Energy Storage Systems, Sol. Energy Mater. Sol. Cells, 2011, 95, p 3341-3346
A.G. Fernandez, S. Ushak, H. Galleguillos, and F.J. Perez, Development of New Molten Salts with LiNO3 and Ca(NO3)2 for Energy Storage in CSP Plants, Appl. Energy, 2014, 119, p 131-140
J.T. Wang, M.Z. Lai, H.J. Han, Z.Y. Ding, S.J. Liu, and D.W. Zeng, Thermodynamic Modeling and Experimental Verification of Eutectic Point in the LiNO3-KNO3-Ca(NO3)2 Ternary System, J. Therm. Anal. Calorim., 2015, 119(2), p 1259-1266
E. Janecke, The Quaternary System Na, K, Ca, Mg//NO3 and its Subsystems, Z. Elektrochem. Angew. Phys. Chem., 1942, 48(9), p 453-467, in German
P.I. Protsenko and A.G. Bergman, Ternary System of Fused Nitrates of Calcium, Potassium, and Sodium, Russ. J. Inorg. Chem., 1950, 20, p 1365-1375, in Russian
Georig & CO GmbH & Co KG. Use of a Ternary Mixture of Salts as a Heat Transmitting Medium and/or as a Heat Storage Medium, Germnan: DE3038844, 1982-04-29 in German
A. Gil, M. Medrano, I. Martorell, A. Lazaro, P. Dolado, B. Zalba, and L.F. Cabeza, State of the Art on High Temperature Thermal Energy Storage for Power Generation. Part 1—Concepts, Materials and Modellization, Renew. Sustain. Energy Rev., 2010, 14(1), p 31-55
A. Lehrman and D. Breslow, The Liquidus Surface of the System Sodium, Lithium and Calcium Nitrates, J. Am. Chem. Soc., 1938, 60(4), p 873-876
A.V. Storonkin, I.V. Vasilkova, and V.I. Shamko, Phase Diagram for the Sodium, Calcium, Lithium DVR Nitrate System, Vopr. Termodin. Geterogen. Sist. i, 1973, 2, p 128-139 (in Russian)
Z.Y. Qiao, X.L. Xing, and M. Peng, Thermodynamic Criterion for Judging the Symmetry of Ternary Systems and Criterion Applications, J. Phase Equilib. Diffus., 1996, 17(6), p 502-507
D. Mantha, T. Wang, and R.G. Reddy, Thermodynamic Modeling of Eutectic Point in the LiNO3-NaNO3-KNO3-NaNO2 Quaternary System, Sol. Energy Mater. Sol. Cells, 2013, 118, p 18-21
T. Wang, D. Mantha, and R.G. Reddy, Thermodynamic Properties of LiNO3-NaNO3-KNO3-2KNO3∙Mg(NO3)2 System, Thermochim. Acta, 2013, 551, p 92-98
Q. Peng, X.X. Yang, J. Ding, X.L. Wei, and J.P. Yang, Thermodynamic Performance of the NaNO3-NaCl-NaNO2 Ternary System, J. Therm. Anal. Calorim., 2014, 115(2), p 1753-1758
G.W. Toop, Predicting Ternary Activities Using Binary Data, Trans. AIME., 1965, 233, p 850-855
I. Ansara, C. Bemard, L. Kaufman, and P. Spencer, A Comparison of Calculated Phase Equilibriums in Selected Ternary Alloy Systems Using Thermodynamic Values Derived from Different Models, CALPHAD, 1978, 2(1), p 1-15
P.P. Lin, A.D. Pelton, and C.W. Bale, Computation of Ternary Molten Salt Phase Diagrams, J. Am. Ceram. Soc., 1979, 62(7-8), p 414-422
M. Hillert, Empirical Methods of Predicting and Representing Thermodynamic Properties, CALPHAD, 1980, 4(1), p 1-12
J. Sangster and A.D. Pelton, Thermodynamic Calculation of Phase Diagrams of the 60 Common-Ion Alkali Halide Ternary Systems Containing Cations Li, Na, K, Rb, Cs and Anions F, Cl, Br, I, J. Phase Equilib., 1991, 12(5), p 511-537
Y. Dessureault, J. Sangster, and A.D. Pelton, Critical Evaluation of Thermodynamic Data and Phase Diagrams of the Hydroxide-Halide, Hydroxide-Nitrate, Nitrate-Nitrate, Nitrate-Halide and Hydroxide-Hydroxide Systems (AOH-AX, ANO3-AX, ANO3-BNO3, AOH-BOH) Where A, B = Li, Na, K and X = Cl, F, NO3, OH, J. Chem. Phys., 1990, 87(3), p 407-453, in French
J. Lumsden, Thermodynamics of Molten Salt Mixtures, Academic Press, London, 1966, p 183-255
J.W. Raade and D. Padowitz, Development of Molten Salt Heat Transfer Fluid with Low Melting Point and High Thermal Stability, J. Sol. Energy Eng., 2011, 133, p 031013-1-031013-6
Acknowledgments
This work is financially supported by Qinghai Science & Technology Department of China under the contract number 2012-H-804.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Xu, F., Han, H. et al. Thermodynamic Modeling and Experimental Verification of Eutectic Point in the LiNO3-NaNO3-Ca(NO3)2 Ternary System. J. Phase Equilib. Diffus. 36, 606–612 (2015). https://doi.org/10.1007/s11669-015-0412-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-015-0412-4