Abstract
Bismuth vanadate (BiVO4) offers high photon efficiencies in solar photo-anodes, due to its suitable semiconductor band gap energies and associated visible light absorption. In well-tuned conditions, such anodes enable green hydrogen generation in photoelectrochemical water splitting cells. Bismuth vanadate films have to ensure high efficiencies in electron/hole pair generation and sufficiently high rates of charge transfer to the conducting substrate and the electrolyte, respectively. Thus, the tuning of coating properties has to aim for high phase purity, good layer integrity as well as optimum diffusion path lengths. In order to explore the potential of aerosol deposition to produce BiVO4 films with high photoelectrochemical activity and to elucidate influences on microstructure and application properties, powder sizes and spraying parameters had to be tailored. By ball milling over durations of up to 20 min, particles sizes in the range from 8.3 down to 0.6 µm were obtained. With respect to spray conditions, the process gas pressure was varied from 1.0 to 2.1 bar corresponding to gas flow rates of 10-40 l/min. The wide range of powder sizes and parameters in aerosol deposition allowed for developing a window of deposition in order to derive the most promising combinations for layer build-up. Optimum parameter sets in application on stainless steel substrates were transferred to FTO-coated glass substrates for backlit cell layouts. The thickness and conductivity of the layers were adjusted to a layer thickness range of 200-500 nm in order to achieve maximum photocurrents. The production of homogeneous, large-scale prototypes demonstrates that aerosol deposition is suitable for processing layers for solar energy harvesting with high photo current densities of up to 3.55 mA/cm2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to stop the rise in global temperature by high CO2 emissions, it is essential to develop alternative solutions that ideally allow for direct and efficient sunlight harvesting and the production of green fuels for energy storage. Hydrogen-based technologies could fulfill both needs. As a renewable energy carrier, hydrogen can be stored in pressurized gas tanks, as cryogenic liquid or in solid metal hydrides (Ref 1). Hydrogen can then be used as a flexible energy carrier in various applications, e.g., to generate electrical energy in fuel cells or to produce ammonia or synthetic hydrocarbons in chemical reactors without any greenhouse gas emissions (Ref 2, 3). Apart from using wind power or solar cells as energy sources to drive water splitting by electrolysis, environmentally friendly hydrogen production can also be achieved by direct conversion of sunlight in a photovoltaic cell. Corresponding photoelectrochemical water splitting cells are based on semiconductor materials to generate charge carriers by the absorption of sunlight, thus enabling the electrolysis reaction and the generation of hydrogen directly from water (Ref 4,5,6). Advantages of such direct conversion over electrolysis concern lower ohmic losses and thus higher efficiencies.
As a metal oxide semiconductor with a band gap energy of 2.4 eV, bismuth vanadate (BiVO4) shows light absorption within the visible range of the solar spectrum, which predestines this material for the use in photoelectrochemical water splitting cells (Ref 7). Moreover, the band gap structure suits well for use as photoanodes for the oxygen evolution reaction (OER). As commercially available low cost and non-toxic material with a good electrochemical stability, BiVO4 should allow for easy technological implementation (Ref 8,9,10).
So far, photo-active films are mainly produced by thin film techniques, but at rather high costs and low deposition rates (Ref 11, 12). Less costly processing routes are opened by thermal spraying (Ref 13) or sol–gel techniques (Ref 14); however, these cannot guarantee the required phase purity or absence of remnants from the binder. As solid state and binderless alternative, aerosol deposition (AD) offers several advantages: comparatively low costs, high deposition rates, as well as absence of undesired phase transformations and impurities or residues that could reduce the photoelectrochemical activity. The approach of this research concerns a systematic investigation in tuning aerosol deposition of BiVO4 coatings for highly effective sunlight harvesting and green hydrogen generation by studying influences of powder sizes and process gas pressures on layer formation.
The aerosol deposition method (AD) was developed in the 1990s by Jun Akedo´s group at the National Institute of Industrial Science and Technology (AIST) in Tsukuba, Japan (Ref 15, 16). Thin ceramic layers can be deposited at room temperature in a vacuum by using small, about micron-sized ceramic particles by spraying onto polymers, metals and ceramics as substrate material. So far, a wide variety of different ceramics has been explored for possible use as spraying material to produce thin ceramic coatings by aerosol deposition (Ref 17). Wolpert et al. demonstrated the feasibility for using aerosol deposition to produce thin BiVO4 coatings for solar hydrogen generation. However, so far a systematic investigation to derive most effective feedstock sizes and spray parameter sets for aerosol deposition of BiVO4, ensuring the needed process stability, is still missing (Ref 18).
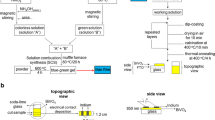
Figure 1 shows a principle sketch of the aerosol deposition method. The aerosol is produced in an aerosol generator and fed directly into the main process gas stream. Due to the flow through a converging nozzle and the gas expansion into a vacuum chamber, the aerosol is accelerated to high velocities of up to 350 m/s (Ref 19). The vacuum chamber is necessary to minimize interaction with the surrounding atmosphere and to reduce the bow shock effect in front of the substrate, thus preventing the fine powder from being deflected with the gas flow.
In general, ceramic particles are brittle and show no plastic deformation. However, according to results from Calvié et al., plastic deformation can be observed even in brittle ceramic particles if they are nano-sized (Ref 20). The bonding mechanisms for aerosol deposition are still controversially discussed (Ref 21). However, literature agrees well with the view that coating formation is associated with substantial grain refinement upon impact (Ref 15). More information about the aerosol deposition can be found in the overview article of Hanft et al. (Ref 17).
Materials and Experimental Procedures
Materials and Preparation
The BiVO4 powder was delivered by American Elements, Los Angeles, California, USA with a particle size of D10 = 2.5 µm, D50 = 8.3 µm and D90 = 17.2 µm. The as-received powder was milled with a planetary mill (Pulverisette 5/4 classic line) from Fritsch GmbH, Idar-Oberstein, Germany, by using 500 ml zirconia grinding vials and 2 mm zirconia grinding balls. Milling was performed at 400 revs/min in isopropanol and was interrupted in intervals of 1 min with 10 min for cooling. The weight ratio of milling balls, powder and isopropanol was set to 10:2:0.25 (with milling balls: 400 g; powder: 80 g; isopropanol: 20 g). In order to achieve different particle sizes, the milling times were varied from 1 to 20 min. The powders were dried in an ambient atmosphere for 2 h at 200 °C, then cooled down in a vacuum and stored in a glove box to prevent the absorption of water before spraying.
In order to develop optimum powder sizes and conditions for deposition, stainless steel AISI 304 plates with a thickness of 3 mm were used as substrates (lateral size of 10 mm × 10 mm). Substrate surfaces were polished to a maximum roughness of Ra ≤ 1 µm. Previously optimized spray conditions were used for processing thin films onto conductive fluorine doped tin oxide (FTO) coated glass substrates from Solaronix SA, Aubonne, Switzerland. FTO substrates allow for backside illumination during photoelectrochemical analyses. The different substrate types were all cleaned with ethanol for 5 min in an ultrasonic bath. Possibly remaining adsorbates on the surface were removed by plasma cleaning in an oxygen atmosphere for 5 min directly before the use in aerosol deposition.
Aerosol Deposition (AD)
Aerosol deposition was performed by using specially designed equipment for ensuring well-defined and reproducible process gas and chamber pressures. The BiVO4 powder was sprayed via aerosol deposition in the as-received and the differently milled states. Nitrogen was used as common carrier and process gas at gas flows of 10, 20 and 40 l/min, corresponding to process gas pressures of 1.0, 1.3 and 2.1 bar, respectively. The base pressure of the vacuum chamber was kept at about 0.3 mbar. The spraying distance was set to 30 mm and the substrates passed the aerosol gas stream with a traverse speed of 15 mm/s. The substrates were coated with two layers to explore the most suitable parameter regime. In order to ensure homogeneous surface coverage, a De Laval slit nozzle was used with nozzle throat dimensions of 4.0 mm × 0.2 mm. The RBG 1000ISD from Palas GmbH, Karlsruhe, Germany was used as powder feeder. The feed rate was adjusted to about 3.8 cm3/h. For selected experiments with fine powders, the cyclone types Z1000 and Z500 from Palas GmbH, Karlsruhe, Germany were used together in line to remove possible agglomerates from the aerosol. The Z1000 can cut off particles larger than 10 µm and the Z500 cut sizes larger than 5 µm.
Powder Size Analyses and Microscopy
The particle size distributions were measured by laser scattering using a LA-910 from Horiba, Kyoto, Japan. The powders were dispersed in distilled water for the particle size distribution analyses.
Powder morphologies, coating microstructures and thickness were examined by high-resolution scanning electron microscopy (HR-SEM) using a dual-beam (electron and ion beam) Helios G4 UC from FEI, now Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA. The acceleration voltage was adjusted to 10 kV. The different cross sections were prepared in situ in the HR-SEM by using the focused Ga+-ion beam of the Helios G4 UC.
The SEM cross section micrographs were used for measuring the coating thickness by using the analyzing tools of the HR-SEM Helios G4 UC. A minimum of five measurements were performed to determine the mean coating thickness.
Post Treatments for Photoelectrochemical Cell Applications
The as-prepared coatings by aerosol deposition contain a number of nanoscaled defects that act as active centers for electron–hole recombination and thus reduced half-cell activities. To further enhance the electrode performance a dilute solution of Ammonium metatungstate hydrate (Sigma Aldrich) was spin coated on top of the electrodes. The electrodes were then annealed in air for 2 h at 500 °C to reduce the amounts of defects and to form a few tens nanometre thick WO3 coating on the surface.
Photoelectrochemical Analyses
The photoelectrochemical analyses were performed by using a three-electrode setup in a Zahner PECC-2 cell (Zahner Elektrik GmbH, Kronach, Germany) controlled by a Zahner Zennium Electrochemical Workstation and 0.15 mol citric acid solution (pH7, 0.441 mol NaOH) as the electrolyte. The electrodes were cycled with 25 mV/s under anodic conditions from open circuit potential (OCP) to 1.8 V against the reference of a reversible hydrogen electrode (RHE). The cyclic analyses were performed under dark conditions and illumination. The sample was illuminated through a quartz glass window (0.503 cm2) using a LOT solar simulator (Xe arc lamp, with AM1.5G filter, 1000 W/m2, OT Quantum Design, Darmstadt, Germany).
Results
Powder Morphologies and Sizes
Figure 2 shows the SEM micrographs of the different BiVO4 powders prepared for aerosol deposition. The morphology of the as-received powder is more or less equiaxed with rather smooth surfaces, only a few particles showing fracture (Fig. 2a). Some particles are agglomerated. High energy milling results in smaller particle sizes (Fig. 2b, c, d, e and f). Already a milling time of 1 min reduces the average particle size by a factor of two from 8.3 to 3.8 µm, resulting in a powder morphology that is more angular and fractured, as is typical for milled ceramic brittle particles (Fig. 2b). By increasing the milling time to 10 min, particle sizes are reduced further (Fig. 2c, d and e). Nevertheless, some coarser particles are retained. Enhancing the milling time to 20 min mainly reduces the amount of larger particles (Fig. 2f). The particle size distributions of the different powders used in this study for aerosol deposition are summarized in Fig. 3. The different mean particle sizes (D50) cover a range from 8.3 (as-received) to 0.6 µm (20 min milled). Particle sizes ranging from 200 nm to 2 µm are claimed as optimum for aerosol deposition as reported in the review by Hanft et al. (Ref 17). By milling techniques, a size distribution with D90 below 2 µm could be only achieved by adjusting the powder mean particle size to 0.6 µm. Such should guarantee a powder size distribution that matches the optimum range for aerosol deposition.
Layer Microstructures: Parameter Optimization on Steel Substrates
Figure 4 shows SEM micrographs of the aerosol sprayed two layer coatings for the different powder size batches and different spray conditions. As shown in the first line of Fig. 4 for the as-received powder with a mean particle size of 8.3 µm, complete surface coverage by deposition of two layers can only be obtained at a gas pressure of 1.3 bar, here reaching a mean coating thickness of approximately 600 nm. Lower or higher gas pressures of 1.0 bar or 2.1 bar lead to non-continuous deposits, in which only fragments of single particles are bound to the substrate surface. The overall layer thickness is significantly smaller than the mean particle size, indicating that particles fracture upon impact.
SEM micrographs of BiVO4 layers processed by aerosol deposition using different particle sizes (lines) and process gas pressures (columns). Continuous layer formation for all gas pressures is only possible for particle sizes < 4 µm. The deposition of bigger particles needs gas pressures higher than 1.3 bar
The following lines in Fig. 4 show examples for layer formation obtained by aerosol spraying of milled powders. The best coating quality with respect to thickness and uniformity was achieved with the 1 min milled powder (D50 = 3.8 µm) at a gas pressure of 1.3 bar. The obtained mean coating thickness reaches 1.5 µm. Further milling of the powder and smaller sizes lead to a reduction of the coating thickness as shown in lines 3-5 of Fig. 4. The milled powders with mean particle sizes from 2.1 to 0.9 µm lead to maximum coating thicknesses at the same process gas pressure of 1.3 bar, however, only reach between 300 and 700 nm under deposition of 2 spray layers.
In summary, for mean particle sizes from 8.3 to 0.9 µm, the coating microstructures presented in Fig. 4 indicate that best conditions for deposition are met at a gas pressure of 1.3 bar. A reduction or an increase of the gas pressure leads to lower coating thicknesses. In contrast, the smallest BiVO4 powder, with a mean particle size of 0.6 µm, milled for 20 min, reaches the highest layer thickness at a gas pressure of 1.0 bar. However, these layers show rather high porosity. For this smallest powder size batch, higher gas pressures reduce the layer thickness from 2.7 (for 1.0 bar) to 1.4 µm (for 1.3 bar) and to 0.6 µm (for 2.1 bar), but also reduce deposit porosities.
Figure 5 shows the coating thickness obtained by 2 layer deposition vs. mean particle size for the three different process gas pressures (1.0 bar ≙ 10 l/min in blue, 1.3 bar ≙ 20 l/min in red, 2.1 bar ≙ 40 l/min in green). For a process gas pressure of 1.0 bar, the diagram clearly illustrates that particle sizes must be smaller than 4 µm to form a coating. However, at higher process gas pressures, coatings can be build-up also for the larger powder size batches.
Coating thickness decreases for particles smaller than 2 µm, as shown in Fig. 4 in the three bottom lines. To some extent, this can be attributed to problems by powder agglomeration during spraying. The milled powders tend to form agglomerates in the aerosol spraying jet or already in the process gas feed line. As reported by Hanft et al. (Ref 17), agglomerates should be loosely bonded to the substrate. Figure 6(a) shows optical macrographs of aerosol sprayed samples with fine powders in size of 0.6 µm directly after the deposition. The yellow spots on the left sample correspond to loosely bound coarse agglomerates. The problems with agglomerates can be solved by using the Palas Z1000 and Z500 cyclones in line after powder feeding. According to the literature (Ref 17), agglomerates can reach high kinetic energies upon impact. However, losses in energy attributed to fracture and separation of primary particles are assumed to lead to porous coatings with insufficient quality. A possible removal of sub-millimeter size layer flakes by delamination at weakly bonded interfaces during deposition could result in locally very thin coated or even uncovered surface areas (craters) as shown in the SEM micrograph in Fig. 6(b). Moreover, loose bonds within just compressed agglomerates may also enhance local layer removal or erosion during ultrasonic bath cleaning of the samples after spraying. The sample on the right side in Fig. 6(a) shows an optical macrograph of an aerosol sprayed sample as obtained under deposition of the small powder in size of 0.6 µm by using both cyclones in line. The homogenous yellow surface color indicates an absence of agglomerates in the spray powder jet. The detailed view by the SEM micrograph in Fig. 6(c) confirms the appearance of a very smooth surface without any craters. This demonstrates the need of using cyclones to separating undesired particle sizes from the aerosol for reaching process stability.
Deposit surfaces with a macrograph of loosely bound agglomerates, (a) left, and a macrograph of a smooth surface by additionally employing cyclones in line for removing agglomerates from the aerosol gas stream, (a) right; a SEM micrograph showing the craters from removed agglomerates after cleaning, (b); and a SEM micrograph showing the smooth surface by using cyclones during spraying, (c), sprayed with the smallest size batch (D50 = 0.6 µm) and a gas pressure of 1.0 bar
Layer Microstructures: Parameter Transfer for Photoelectrochemical Applications
In photoelectrochemical applications, highest efficiencies are achieved, if active coatings are directly illuminated from the backside through the substrate. Such is realized by the use of glass substrates that are coated with fluorine-doped tin oxide (FTO) to ensure electrical conductivity in contact to the BiVO4 layer. In order to avoid losses by electron–hole recombination, coating thickness should be tuned to about 0.5 µm. In addition, the coating should have low porosity, well bonded internal interfaces and a good connection to the substrate in order to avoid ohmic losses and to enable a high half-cell efficiency. These boundary conditions and possible influences of this substrate type on coating build-up call for separate optimization procedures to transfer basic parameter sets to photoelectrochemical cells for hydrogen generation.
Based on the general optimization procedures, the powders with mean particle sizes of 2.1 µm (milled for 2 min) and 0.6 µm (milled for 20 min) were selected for transferring the process to FTO coated glass substrates. Gas pressures were adjusted to 1.3 bar for the larger powder (D50 = 2.1 µm) and to 1.0 bar as well as 2.1 bar for the smaller one, respectively. In case of the finest powder (D50 = 0.6 µm) the line of cyclones was used to remove agglomerates. Powders and pressures were selected according to the best results presented in Fig. 4. The coating thickness is varied by robot traverse speeds and numbers of spray layers. According to Fig. 4, powder mean particle sizes of 3.8 µm result in better layer build-up than sizes of 2.1 µm. However, another set of experiments dealing with the kinematic parameters (not shown here) demonstrate, that this powder is probably to coarse for homogeneous layer build-up. For powder sizes of 3.8 µm, obtained coating thicknesses did not correlate to the different parameters like numbers of spray layers or traverse robot speeds. In contrast, such correlations can be derived for using powder sizes of 2.1 µm. Figure 7 compares typical SEM cross section micrographs of these coatings on FTO glass substrates. Figure 8 summarizes the obtained coating thicknesses for the different kinematic parameters. In addition, the target coating thickness between 200 and 500 nm is indicated (green area). Figure 7(a), (b), (c) and (d) shows cross sections of aerosol sprayed coatings on the basis of the powder with a mean particle size of 2.1 µm as processed with a gas pressure of 1.3 bar. The coatings were processed by applying two layers and traverse speeds of (a) 5 mm/s, (b) 10 mm/s and (c) 20 mm/s. The coating in (d) was produced as one layer by using a traverse speed of 20 mm/s. The SEM micrographs show quite dense and thin coatings with a layer thickness of around 700 nm for a traverse speed of 5 and 10 mm/s. By increasing the traverse speed, the coating thickness decreases down to 400 nm for 20 mm/s. Using a traverse speed of 20 mm/s, and one instead of two layers for deposition, the coating thickness is reduced to 300 nm. In addition, the micrographs reveal that some of the coatings show poor adhesion to the FTO surface of the substrate, as can be seen in Fig. 7(b) and (c).
SEM micrographs of BiVO4 coatings on fluorine-doped tin oxide glass substrates (FTO glass) processed by aerosol deposition using the 2 min milled powder batch (a–d) with a mean particle size of 2.1 µm, a process gas pressure of 1.3 bar and two layers for different traverse speeds (a) 5 mm/s, (b) 10 mm/s, (c) 20 mm/s and (d) one layer at 20 mm/s. SEM micrographs (e) and (f) show BiVO4 coatings on FTO glass processed by aerosol deposition using the 20 min milled powder batch with a mean particle size of 0.6 µm, two layers, traverse speed of 5 mm/s by applying the cyclones for a process gas pressure of (e) 1.0 bar and (f) 2.1 bar
Figure 7(e) and f shows the SEM micrographs of the aerosol sprayed coatings on basis of the small powder with 0.6 µm in size for using a traverse speed of 5 mm/s, two layers and a gas pressure of (e) 1.0 bar and (f) 2.1 bar for deposition. The cross sections reveal very thin coatings with thickness of about 300 nm for a gas pressure of 1.0 bar and around 200 nm for a gas pressure of 2.1 bar, respectively. The overall lesser coating thickness can be attributed to the reduced aerosol load in the gas jet by using the cyclones. The coatings are well bonded to the FTO glass substrate. However, also some local areas are uncoated, which could reduce the half-cell efficiency. A high half-cell efficiency requires complete surface coverage, good coating integrity as well as a coating thickness within a range from 0.2 to 0.5 µm. These conditions are only met by the coatings given in Fig. 7(d) and (e). The BiVO4 coating in Fig. 7(c) is also within the optimum thickness range, but misses the required bonding quality to the substrate.
Upscaling for Photoelectrochemical Cell Production and Efficiencies
The macrograph in Fig. 9 shows a BiVO4 coated 10 cm × 10 cm FTO plate as deposited under an optimized parameter set (D50 = 0.6 µm, p = 1.0 bar, use of cyclones). The semi-translucent appearance against the sunlight visualizes an uniform layer formation. The conductive gold tracks (dark vertical lines) are needed to ensure low ohmic losses in photo-electric cells. Nevertheless, substrate conductivity still needs further improvement on such large scales. The overall appearance with respect to color and translucency given in Fig. 9 shows that aerosol deposition is a suitable tool to coat larger areas homogenously with BiVO4 layers. Figure 10 shows the results of cyclic voltammetry experiments under front illumination with an aerosol sprayed bismuth vanadate electrode sample with dimensions of 15 mm × 30 mm after thermal treatment (see “Post Treatments” above). During the analyzes, a circular area (diameter 8 mm) was exposed against the electrolyte. Under this condition, the samples has a photo current density of 3.55 mA/cm2.
Macrograph showing a homogenous semitransparent BiVO4 coating on a 10 × 10 cm FTO glass substrate plate under illumination by sunlight. The coating was produced by using a mean powder size of 0.6 µm, a process gas pressure of 1.0 bar and both cyclones in the powder feed line. The darks stripes (vertical lines) corresponds to conductive gold tracks to ensure good conductivity in electro-chemical cells
Cyclic voltammetry (only the first upward cycles are shown here) of an aerosol deposited BiVO4 electrode after thermal treatment and CoPi decoration under AM1.5G illumination (blue) and in the dark (black). At the water splitting potential of 1.23 V, a photo current density of 3.55 mA/cm2 is obtained under illumination. The experiment was performed with front illumination under pH7 in citrate electrolyte that act as hole scavenger
Discussion
The results for powder size and parameter variation allow the development of a process map of successful BiVO4 coating formation by aerosol deposition. Successful coating formation is here defined by a coating thickness of 250 nm as a threshold for continuous layer formation on steel substrates without using cyclones in the powder line. The resulting process map is given in Fig. 11 and distinguishes regimes of coating formation from regimes of fracture (no coating). This process map also includes data for 1.1 bar (15 l/min) and 1.7 bar (30 l/min), which have been omitted in the selection of Fig. 4. The comparison shows that all powder sizes allow for successful coating formation. However, in the case of the larger powders, particularly the as-received powder with a mean particle size of 8.3 µm, it is beneficial to use higher process gas pressures. Figure 11 also shows that successful coating formation can be achieved for all parameter sets applied in this work if the particles are smaller than 4 µm. However, as shown by the micrographs, coating integrity with respect to internal porosity and adhesion to substrate differs, indicating less defects under use of higher process gas pressures of 1.3 and 2.1 bar.
For most examples of the present investigation, the layer thickness is lesser than the mean particle size. This indicates that fragmentation takes place in aerosol deposition of BiVO4, in good agreement with the literature on experimental work that claim size dependent particle fracture and grain refinement as a pre-requisite for bonding (Ref 15, 17). The basic features of aerosol deposition are also supported by simulation results given in the literature. Under the aim to gain a better understanding of the binding phenomena in aerosol deposition, Daneshian et al. performed molecular dynamic (MD) simulations (Ref 22), (Ref 23). Despite the simplified 2-D approach in the model, the simulation results allow to distinguish between (1) rebound events, (2) deformation and bonding as well as (3) particle fracture depending on powder size and velocity during aerosol deposition. They show that particle deformation as well as fracture could contribute to bonding. In line with this view, the critical velocity to reach deformation and bonding may be defined by a threshold to locally exceed sufficiently high degrees of stress and deformation or fracture in the particle interior. The combination of deformation and fracture rather well corresponds to observed results in aerosol deposition of BiVO4 coatings. Figure 12 shows the surface topography of an aerosol sprayed BiVO4 coating with (i) fractured and (ii) deformed particles as well as mixed modes thereof. The fractured particles show (i) the typical fracture pattern of brittle ceramics. Other areas of this SEM picture show deformed and densified particles (ii) as reached under the high particle velocity. These more ductile areas miss signs of brittle BiVO4 failure.
Based on the topographies from Fig. 12, it should be noted here that multiple effects are already present in one layer. This is due to the distribution of particle sizes in every powder batch and individually varying particle impact velocities. In summary, further work is needed to optimize the spraying process or the pre-treatment of the milled powders to reduce the agglomeration in the aerosol stream and to enhance the coating quality. Nevertheless, the process map for aerosol deposition of BiVO4, developed in the present study, might provide a good basis for that.
The gained knowledge using stainless steel substrates could be transferred to other substrate materials. In this study, special focus is given on fluorine-doped tin oxide (FTO) glasses, which are used for applications in back-lit photoelectrochemical cells. First experiments in this work demonstrate that it is possible to coat larger areas of FTO substrates homogeneously by aerosol deposition. A homogeneous coating quality and the required coating thickness of around 200-500 nm is achieved by using well-tuned feed rates and kinematics during deposition. Herein, the results for using FTO glass substrates indicate that the increase in coating thickness not linearly scales with the amount of material deposited onto area units as induced here by slower traverse scan speeds or less layers. In addition, influences from powder sizes and spray parameter sets have to be considered. Thus, the adjustment of a defined coating thickness and quality is more difficult than expected, which could be attributed to effects by deceleration on particle deformation and fracture upon impact, given by impact velocities and response by properties of the present surface to deposit on.
By parameter optimization and guaranteeing the required process stability, aerosol deposition of BiVO4 could be successfully upscaled for processing uniform anodes for photo-electrochemical hydrogen production. Under herein established parameter sets and procedures, photo current densities of about 3.55 mA/cm2 are achieved, which are two times higher than those obtained by samples that are processed by aerosol deposition of non-optimized, as-received powder shown by Wolpert et al. [13]. According to results reported in literature, photo current densities from 1.5 to 2 mA/cm2 were reached by using thin film techniques such as spray pyrolysis or pulsed layer deposition for layer production (Ref 24,25,26,27). Abdi et al. (Ref 24, 28) and Jang et al. (Ref 29) demonstrated that photo current densities of BiVO4 layers, which were prepared by thin film techniques, could be increased to 3.5 mA/cm2. It might be worth noting that such high current densities are only attainable with complex post treatments (Ref 28, 29). In comparison to results from literature, the aerosol deposited BiVO4 coatings of the present study already show a similar photo-electrochemical performance as good layers that were processed by thin film techniques. Nevertheless, aerosol deposited BiVO4 coatings still have a potential for further improvement, for example by doping or by applying co-catalysts layers, which is currently being under investigation. With such efforts, the quality of BiVO4 coating could move a step forward toward the theoretical photo current density of BiVO4 of 7.5 mA/cm2 as estimated for test conditions using a LOT solar simulator with a Xe arc lamp, AM1.5G filter and a power density of 1000 W/m2 (Ref 24).
Summary and Conclusions
The presented, comprehensive investigation allows to distinguish effects of powder sizes and of process parameter sets on successful BiVO4-layer build-up by aerosol deposition. Based on the systematic variation of milled feedstock powder sizes and process gas pressures, a process map of the necessary conditions for successful BiVO4 coating formation is derived. On stainless steel substrates, larger powders need higher gas pressures for successful layer deposition. For all feedstock sizes, higher pressures appear to be beneficial for reducing coating porosity. Layer build-up is associated with particle deformation and fracture, which can result in coating thicknesses smaller than the initial particle sizes. Respective procedures for coating optimization are transferable to other substrate materials such as FTO glass. Applying optimum powder sizes and spray conditions in aerosol deposition as well as tuned kinematics, thin semi-transparent and homogenous layers can be processed in larger scales that fulfill the needs in direct sunlight harvesting, e.g., in photoelectrochemical cells.
References
J. Bellosta von Colbe, J.-R. Ares, J. Barale, M. Baricco, C. Buckley, G. Capurso, N. Gallandat, D.M. Grant, M.N. Guzik, I. Jacob, E.H. Jensen, T. Jensen, J. Jepsen, T. Klassen, M.V. Lototskyy, K. Manickam, A. Montone, J. Puszkiel, S. Sartori, D.A. Sheppard, A. Stuart, G. Walker, C.J. Webb, H. Yang, V. Yartys, A. Züttel, and M. Dornheim, Application of Hydrides in Hydrogen Storage and Compression: Achievements, Outlook and Perspectives, Int. J. Hydrogen Energy, 2019, 44(15), p 7780-7808.
M. Klell, H. Eichlseder, and A. Trattner, Wasserstoff in der Fahrzeugtechnik, Springer Fachmedien Wiesbaden, London, 2018.
S. Sengodan, R. Lan, J. Humphreys, D. Du, W. Xu, H. Wang, and S. Tao, Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications, Renew. Sustain. Energy Rev., 2018, 82, p 761-780.
W.-H. Cheng, M.H. Richter, M.M. May, J. Ohlmann, D. Lackner, F. Dimroth, T. Hannappel, H.A. Atwater, and H.-J. Lewerenz, Monolithic Photoelectrochemical Device for Direct Water Splitting with 19% Efficiency, ACS Energy Lett., 2018, 3(8), p 1795-1800.
Z. Li, W. Luo, M. Zhang, J. Feng, and Z. Zou, Photoelectrochemical Cells for Solar Hydrogen Production: Current State of Promising Photoelectrodes, Methods to Improve their Properties, and Outlook, Energy Environ. Sci., 2013, 6(2), p 347-370.
Z. Zou, J. Ye, K. Sayama, and H. Arakawa, Direct Splitting of Water Under Vsible Light Irradiation with an Oxide Semiconductor Photocatalyst, Nature, 2001, 414(6864), p 625-627.
J.H. Kim and J.S. Lee, BiVO4-Based Heterostructured Photocatalysts for Solar Water Splitting: A Review, Energy Environ. Focus, 2014, 3(4), p 339-353.
Z.-F. Huang, L. Pan, J.-J. Zou, X. Zhang, and L. Wang, Nanostructured Bismuth Vanadate-Based Materials for Solar-Energy-Driven Water Oxidation: A Review on Recent Progress, Nanoscale, 2014, 6(23), p 14044-14063.
F.M. Toma, J.K. Cooper, V. Kunzelmann, M.T. McDowell, J. Yu, D.M. Larson, N.J. Borys, C. Abelyan, J.W. Beeman, K.M. Yu, J. Yang, M.R. Le Chen, J. Shaner, F.A. Spurgeon, K.A. Persson. Houle, and I.D. Sharp, Mechanistic Insights into Chemical and Photochemical Transformations of Bismuth Vanadate Photoanodes, Nat. Commun., 2016, 7, p 12012.
B.-C. Xiao, L.-Y. Lin, J.-Y. Hong, H.-S. Lin, and Y.-T. Song, Synthesis of a Monoclinic BiVO4 Nanorod Array as the Photocatalyst for Efficient Photoelectrochemical Water Oxidation, RSC Adv., 2017, 7(13), p 7547-7554.
P. Brack, J.S. Sagu, T.A.N. Peiris, A. McInnes, M. Senili, K.G.U. Wijayantha, F. Marken, and E. Selli, Aerosol-Assisted CVD of Bismuth Vanadate Thin Films and Their Photoelectrochemical Properties, Chem. Vapor Depos., 2015, 21(1-3), p 41-45.
A.N. Bondarchuk, I. Corrales-Mendoza, J.A. Aguilar-Martínez, S.A. Tomás, D.A. Gómez-Caiceros, A. Hernández-Méndez, and F. Marken, A BiVO4 Photoanode Grown on Porous and Conductive SnO2 Ceramics for Water Splitting Driven by Solar Energy, Ceram. Int., 2020, 46(7), p 9040-9049.
S.K. Holland, M.R. Dutter, D.J. Lawrence, B.A. Reisner, and T.C. DeVore, Photoelectrochemical Performance of W-Doped BiVO4 Thin-Films Deposited by Spray Pyrolysis, Proceedings of SPIE—The International Society for Optical Engineering Solar Hydrogen and Nanotechnology Viii. Y. Kanai, D. Prendergast Ed., SPIE, San Diego, 2013
S. Hilliard, D. Friedrich, S. Kressman, H. Strub, V. Artero, and C. Laberty-Robert, Solar-Water-Splitting BiVO4 Thin-Film Photoanodes Prepared By Using a Sol–Gel Dip-Coating Technique, ChemPhotoChem, 2017, 1(6), p 273-280.
J. Akedo, Aerosol Deposition of Ceramic Thick Films at Room Temperature: Densification Mechanism of Ceramic Layers, J. Am. Ceram. Soc., 2006, 89(6), p 1834-1839.
J. Akedo, Room Temperature Impact Consolidation (RTIC) of Fine Ceramic Powder by Aerosol Deposition Method and Applications to Microdevices, J. Therm. Spray Technol., 2008, 17(2), p 181-198.
D. Hanft, J. Exner, M. Schubert, T. Stöcker, P. Fuierer, and R. Moos, An Overview of the Aerosol Deposition Method: Process Fundamentals and New Trends in Materials Applications, J. Ceram. Sci. Technol., 2015, 06(3), p 147-182.
C. Wolpert, T. Emmler, M. Villa Vidaller, A. Elsenberg, K. Shinoda, M. Schieda, F. Gärtner, J. Akedo, and T. Klassen, Aerosol-Deposited BiVO4 Photoelectrodes for Hydrogen Generation, J. Therm. Spray Technol., 2021, 30(3), p 603-616.
A. Elsenberg, F. Gärtner, and T. Klassen, Aerosol Deposition of Ti3SiC2-Max-Phase Coatings, J. Therm. Spray Technol., 2021, 30(5), p 1121-1135.
E. Calvié, J. Réthoré, L. Joly-Pottuz, S. Meille, J. Chevalier, V. Garnier, Y. Jorand, C. Esnouf, T. Epicier, J.B. Quirk, and K. Masenelli-Varlot, Mechanical Behavior Law of Ceramic Nanoparticles from Transmission Electron Microscopy In Situ Nano-Compression Tests, Mater. Lett., 2014, 119, p 107-110.
K. Shinoda, F. Gaertner, C. Lee, A. Dolatabadi, and S. Johnson, Kinetic Spraying of Brittle Materials: From Layer Formation to Applications in Aerosol Deposition and Cold Gas Spraying, J. Therm. Spray Technol., 2021, 30(3), p 471-479.
B. Daneshian, F. Gaertner, H. Assadi, D. Hoeche, W. Weber, and T. Klassen, Size Effects of Brittle Particles in Aerosol Deposition—Molecular Dynamics Simulation, J. Therm. Spray Technol., 2021, 30(3), p 503-522.
B. Daneshian, F. Gärtner, H. Assadi, M.V. Vidaller, D. Höche, and T. Klassen, Features of Ceramic Nanoparticle Deformation in Aerosol Deposition Explored by Molecular Dynamics Simulation, Surf. Coat. Technol., 2022, 429, p 127886.
F.F. Abdi, N. Firet, and R. van de Krol, Efficient BiVO4 Thin Film Photoanodes Modified with Cobalt Phosphate Catalyst and W-doping, ChemCatChem, 2013, 5(2), p 490-496.
H. Khan, M.-J. Kim, J.-H. Baek, S. Bera, H.-J. Woo, H.-S. Moon, and S.-H. Kwon, Sustained Water Oxidation with Surface- and Interface-Engineered WO3/BiVO4 Heterojunction Photoanodes, ACS Appl. Energy Mater., 2022, 5(12), p 15788-15798.
D. Lee, W. Wang, C. Zhou, X. Tong, M. Liu, G. Galli, and K.-S. Choi, The Impact of Surface Composition on the Interfacial Energetics and Photoelectrochemical Properties of BiVO4, Nat. Energy, 2021, 6(3), p 287-294.
D.K. Zhong, S. Choi, and D.R. Gamelin, Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by “Co-Pi” Catalyst-Modified W:BiVO4, J. Am. Chem. Soc., 2011, 133(45), p 18370-18377.
F.F. Abdi, L. Han, A.H.M. Smets, M. Zeman, B. Dam, and R. van de Krol, Efficient Solar Water Splitting by Enhanced Charge Separation in a Bismuth Vanadate-Silicon Tandem Photoelectrode, Nat. Commun., 2013, 4, p 2195.
J.W. Jang, D. Friedrich, S. Müller, M. Lamers, H. Hempel, S. Lardhi, Z. Cao, M. Harb, L. Cavallo, R. Heller, and R. Eichberger, Enhancing Charge Carrier Lifetime in Metal Oxide Photoelectrodes Through Mild Hydrogen Treatment, Adv. Energy Mater., 2017, 7(22), p 1701536.
Acknowledgments
The authors like to thank Charline Wolpert for fruitful discussions and the laboratory staff of the Helmut Schmidt University, Thomas Breckwoldt, Marion Kollmeier, Stefan Meyer, Matthias Schulze and Camilla Schulze for support in experimental work. The authors also like to thank Dagmara Dowbor from the Helmut Schmidt University for proofreading of the English style.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is an invited paper selected from presentations at the 2022 International Thermal Spray Conference, held May 4–6, 2022 in Vienna, Austria, and has been expanded from the original presentation. The issue was organized by André McDonald, University of Alberta (Lead Editor); Yuk-Chiu Lau, General Electric Power; Fardad Azarmi, North Dakota State University; Filofteia-Laura Toma, Fraunhofer Institute for Material and Beam Technology; Heli Koivuluoto, Tampere University; Jan Cizek, Institute of Plasma Physics, Czech Academy of Sciences; Emine Bakan, Forschungszentrum Jülich GmbH; Šárka Houdková, University of West Bohemia; and Hua Li, Ningbo Institute of Materials Technology and Engineering, CAS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsenberg, A., Emmler, T., Schieda, M. et al. Tuning Aerosol Deposition of BiVO4 Films for Effective Sunlight Harvesting. J Therm Spray Tech 32, 352–362 (2023). https://doi.org/10.1007/s11666-023-01550-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-023-01550-0