Abstract
Hydrogen generation from renewable energy sources will play a key role in the concerted endeavor to constrain climate change. One environmentally friendly route, powered by sunlight, is the photoelectrochemical water splitting cell (PEC). This technology employs electrodes coated with thin films of semiconductor materials to capture light and generate charge carriers that directly drive the water splitting reaction. Bismuth vanadate is a promising metal oxide semiconductor, as it absorbs visible light, and is abundant, non-toxic and cost-effective. The present study investigates the formation of bismuth vanadate thin films by the aerosol deposition (AD) method. Operating with layer formation at room temperature, AD offers advantages over other routes for the fabrication of photoactive thin film coatings, as no binders or sintering processes need to be applied. Furthermore, compared to traditional cold spraying, micrometer-sized particles can be used, resulting in coatings with thicknesses below 1 µm. Additionally, the lower kinetic energy of the feedstock powder particles enables the use of delicate substrates, such as FTO-coated glass, expanding the range of possible PEC device configurations. The process parameters explored in this study had considerable influence on the resulting coating microstructure, which in turn showed a significant impact on the photoelectrochemical performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analysis of world climate data and its correlation to human activity shows that the use of fossil fuels must be reduced drastically in order to restrict emissions of greenhouse gases to a minimum. Renewable energy sources and storage systems have therefore gained increasing attention from scientists worldwide (Ref 1, 2). Hydrogen qualifies especially as a renewable energy carrier and can be used in fuel cells to generate electrical energy with zero emissions, or in chemical reactors to produce, for example, ammonia from nitrogen, or synthetic hydrocarbons from carbon dioxide (Ref 3,4,5). Hydrogen can be stored over long periods of time in pressurized tanks, or in the form of chemical compounds such as metal hydrides, which allows for both stationary and mobile applications. An environmentally friendly production route for hydrogen is via the electrolysis of water (electrochemical water splitting), particularly if the necessary electrical current is obtained from a renewable energy source, such as a photovoltaic cell. Photoelectrochemical water splitting cells are devices in which charge carriers, generated through light absorption in a semiconductor material, are used to drive the electrolysis reaction, enabling the production of hydrogen directly from water and sunlight (Ref 6,7,8).
The metal oxide semiconductor bismuth vanadate (BiVO4), in its monoclinic phase, is considered a promising light absorbing material for solar-driven water splitting. Its band gap of 2.4 eV results in light absorption within the visible range of the solar spectrum, and its electronic structure (specifically, the position of its band edges) allows for implementation in photoanodes for the oxygen evolution reaction (OER). Moreover, BiVO4 is a non-toxic and low-cost material with good stability in electrochemical conditions (Ref 9,10,11).
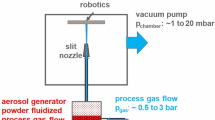
The present study investigates whether the aerosol deposition (AD) method can be employed to coat conducting substrates with a thin bismuth vanadate (BiVO4) layer, with properties adequate for efficient photoelectrochemical water splitting. As invented by J. Akedo in the 1990s (Ref 12,13,14), AD is a solvent-free solid state kinetic spraying method for the deposition of small-sized ceramic particles onto various substrate materials, usually operating at room temperature. At present, it provides solutions for a wide field of applications (Ref 12, 15,16,17). Figure 1 illustrates the principle.
In AD, the fine feedstock powder is dispersed in a carrier gas stream inside an aerosol generation unit. The fluidized aerosol–gas stream is accelerated during the flow through a nozzle toward the substrate in a vacuum deposition chamber. The pressure difference between the nozzle inlet and the vacuum chamber causes expansion and acceleration of the process gas, by its high velocity providing the required kinetic energy for successful particle impact onto the substrate. Due to the small sizes of the powder particles, it can be assumed that the deformation behavior is different to that of bulk ceramics. However, details about bonding mechanisms have not been fully clarified yet (Ref 18).
The application of AD-sprayed coatings is a new way to obtain stable functional thin layers on nearly any type of substrate material. In particular, fluorine-doped tin oxide (FTO)-coated glass substrates are widely used when investigating material systems for photoelectrochemical applications, since they can be integrated into a wide range of device configurations. For example, they can be implemented with backside illumination, which, in the case of electron-transport limited absorbers such as BiVO4, allows more photogenerated electrons to reach the back contact, thus minimizing recombination losses.
Coatings of BiVO4 on glass/FTO substrates can also be made via the well-established doctor blade method for direct coating with BiVO4 (nano-)particles, or via mixing of bismuth and vanadium precursor solutions that are spin-coated or jet-printed, and thermally treated afterwards (Ref 19, 20). The spray pyrolysis method combines deposition and reaction by spraying the precursor solution mixture on a heated substrate (Ref 21). Furthermore, nanostructured BiVO4 coatings can be directly grown on glass/FTO substrates by controlling the temperature of the precursor mixture, as demonstrated by Xiao et al. (Ref 11). However, the doctor blade method has very limited scalability, and the precursor-based methods cannot be used to implement previously synthesized particles with optimized chemical composition.
In order to evaluate the possible application potential, the functional properties of the AD-sprayed coatings are compared to those of samples processed by cold spraying (CS) serving here as reference method. CS is a kinetic spraying method operating with solid state impacts at temperatures below the materials melting point and is usually applied for ductile materials. In CS, the solid powder particles are accelerated in a heated high-pressure gas stream to velocities of typically 400-800 m/s. The particles experience ultra-fast, severe plastic deformation upon impact and by thermal softening and associated adiabatic shear instabilities bond to the substrate or form a coating with already deposited layers (Ref 22, 23). As reported in the literature for anatase TiO2, CS can be successfully applied to produce photoactive material coatings (Ref 24,25,26,27,28,29).
The present study describes bismuth vanadate coating microstructures and cohesion properties and takes a first look at the photoelectrochemical performance of BiVO4 layers deposited using AD and CS techniques.
Material and Experimental Procedures
Materials
Commercially available BiVO4 powder (AE1), obtained from American Elements, Los Angeles, California, USA, was used as feedstock material for the AD process of this study. Its mean particle size is approximately 5 µm. In order to remove adsorbed water and avoid agglomeration of the powder particles, thus reaching a better flowability, as well as to reduce defects in the crystal structure, different pre-heat treatments in air before spraying were tested. For CS, a commercially available BiVO4 powder (AA) was used, obtained from Alfa Aesar, Kandel, Germany. Mean particle sizes are approximately 19 µm for AA. The larger particle size of this powder minimizes bow-shock effects and thus provides superior properties for cold gas spraying as compared to AE1. The powder was applied for coating manufacturing without prior heat treatment.
Titanium plates (30 × 30 mm) were used as substrate material for the CS coatings. The high kinetic energy achieved by the powder particles in this process enables an optimal bonding to the metallic substrate. However, the high inertia of cold-sprayed particles would cause severe damage or erosion to brittle substrate materials, thus limiting the variety of possible applications. AD, on the other hand, is a milder deposition method, which allows for more delicate substrates. In this work, fluorine-doped tin oxide (FTO)-coated glass plates were used as substrate materials for the AD experiments. Transparent supports open the possibility for backside illumination, as discussed below, and furthermore enable the construction of stacked devices, with additional layers to capture the fraction of sunlight that is not absorbed in the uppermost coating.
The titanium plates were polished until reaching a maximum surface roughness Ra of ≤ 1 µm. For the as-delivered FTO-coated glass substrates, no further surface modification was applied. Prior to deposition, all substrates were cleaned in an ultrasonic bath by using distilled water and ethanol for 15 min each and additionally plasma etched in oxygen atmosphere for 5 min to remove possible adsorbates.
The particle size distribution was measured by laser scattering using an instrument type LA-910 from Horiba, Kyoto, Japan. Particle morphologies were examined by high-resolution scanning electron microscopy (SEM) using a Helios G4 UC from FEI, now Thermo Fisher Scientific Inc, Waltham, Massachusetts, USA.
Aerosol Deposition (AD) Method
The BiVO4 powder (AE1) was sprayed by AD in the as-received state as well as after the application of two different heat treatments. A brief description of the aerosol deposition process is given in the introduction together with the schematic in Fig. 1. Further descriptions on influences by parameter sets are given in the literature (Ref 30). All samples were prepared with a lab-built aerosol deposition equipment using nitrogen as carrier gas stream at gas flow rates between 7 l/min and 25 l/min to ensure gas velocities close to the speed of sound. For the experiments, slit nozzle exit widths ranging from 4 to 10 mm were used. All coatings were processed as single layer to minimize deposit thickness. After the spraying process, the AD samples were annealed in air for 2 h at 500 °C to guarantee better cohesion within the coating as well as improved adhesion to the substrate. Respective spraying parameters are summarized in Table 1.
Cold Spraying (CS)
Cold spraying was processed with the HSU prototype equivalent to the Kinetiks 8000 equipment from CGT GmbH, Ampfing, Germany, now Oerlikon Metco, Switzerland. The cold-sprayed samples were as well annealed for 2 h in air at 500 °C. The spraying parameters were optimized in previous investigations and correspond to those being optimum for cold spraying of anatase TiO2 (Ref 27, 28). The CS parameters of present investigation are summarized in Table 2.
Microstructure Characterization
The dual-beam device Helios G4 UC, combining electron microscopy and focused ion beam milling, was used to examine the microstructures and coating thicknesses of the coated samples. Ga+ ions of the focused ion beam were used for milling in order to prepare cross sections of the coatings and thin cross-sectional lamellas for analysis in the scanning transmission electron microscopy (STEM) mode.
Cavitation Testing
Cavitation occurs under the attack of fast collapsing gas bubbles in a fluid flow after formation in local pressure gradients. In the present study, defined cavitation testing was used to describe cohesive strengths of the AD-sprayed coatings, thus providing information on the coating integrity. The tests were conducted according to the ASTM-standard G32-10 using a testing equipment type BK101Z from KLN Ultraschall GmbH, Heppenheim, Germany. A vibrating counter body mounted onto a sonotrode was located at a distance of 0.5 mm from the sample surface. This configuration was immerged into distilled water. The sonotrode operated at a frequency of 20 kHz and an amplitude of 50 µm. After each cavitation step, mass losses were measured and respective surface damages were analyzed by scanning electron microscopy (SEM) using a Quanta 650 from FEI, now Thermo Fisher Scientific Inc, Waltham, Massachusetts, USA.
Photoelectrochemistry
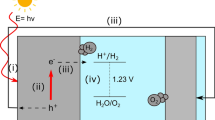
Photoelectrochemical (PEC) characterization was performed in a three-electrode cell (Zahner PECC2), controlled by a Zennium electrochemical workstation (ZAHNER-Elektrik GmbH & Co.KG, Kronach, Germany). The films under study were contacted as working electrodes. A platinum wire was used as a counter electrode, and an Ag/AgCl (3.0 M NaCl) reference electrode (+ 209 mV versus NHE) was used for measurements in neutral media (pH7 phosphate buffer). The samples were illuminated through a quartz window (2.544 cm2) using a solar simulator (Xe arc lamp, with AM1.5G filter, 1000 W/m2, LOT Quantum Design, Darmstadt, Germany). PEC measurements were also performed with addition of sodium sulfite (Na2SO3) to the electrolyte as hole scavenger. The principle of the PEC cell is sketched in Fig. 2.
After assembling the photoelectrochemical cell, the electrodes were left to equilibrate with the electrolyte and to establish their open-circuit potential (OCP). During the PEC experiments, the potential was cycled three to five times from the OCP to 1.8 V versus RHE (1.5 V versus RHE with added Na2SO3) and back operating at a scan rate of 25 mVs-1.
Results
Powder Morphology
Figure 3 shows the morphologies of the different powders (a, b, c) and the respective cumulative size distributions (d). According to laser scattering analysis, the as-received BiVO4 powder used for AD coatings (AE1) (Fig. 3a) had particle sizes of d10 = 0.4 µm, d50 = 4.0 µm and d90 = 9.1 µm. The particles were rather big in comparison with powder sizes typically used in AD (Ref 31). As milled powders, they showed an irregular equiaxed shape with partly ridged surface and fractured sites. To evaporate adsorbed water from the surface, and to heal defects in the crystalline structure, thus improving the charge carrier transport properties, the powder was heat-treated for 2 h at 500 °C directly before the deposition experiments. The resulting powder morphologies are shown in Fig. 3(b). As compared to the as-received state, particles of the annealed powder appeared to be less angular with rather soft edges. Laser scattering revealed particle sizes of d10 = 1.9 µm, d50 = 6.0 µm and d90 = 12.5 µm indicating that particle sizes grow during the powder annealing. According to the cumulative size distribution given in Fig. 3(d), the size increase is more prominent for smaller particles. The BiVO4 powder provided by Alfa Aesar (AA) (Fig. 3c), used for CS, shows a wide particle size distribution of d10 = 1.7 µm, d50 = 18.8 µm and d90 = 54.8 µm. These powder particles typically show irregular shapes, partly aggregated into big clusters.
SEM micrographs of the BiVO4 feedstock powder: The powder from American Elements (AE1) used for AD is shown in as-received state (a) and after heat treatment for 2 h at 500 °C (b). (c) shows the Alfa Aesar (AA) used for CS and (d) plots of the cumulative particle size distribution for all three powders
Global Coating Appearance
Figure 4 shows overviews of aerosol-sprayed BiVO4 layers on FTO-coated glass substrates. Both samples were sprayed at the same AD conditions using a 10-mm slit nozzle and a carrier gas flow of 10 l/min as standard parameter set for AD. The only distinction between both concerns the heat pre-treatment of the powder. Figure 4(a) presents a top view of an AD-processed BiVO4 coating, produced with powder that was heat-treated for 12 h at 200 °C in air atmosphere. Figure 4(b) shows a top view of an AD-sprayed BiVO4 coating using a powder heat treatment of 2 h at 500 °C in air atmosphere before deposition experiments. The sample (a) locally exhibits large differences in the amounts of deposited powder on the substrate, as demonstrated by lighter colored areas of low surface coverage, and more intense colored areas of complete layer formation. The coating shows a regular pattern that results from the inhomogeneous powder feeding and the line guidance of the robot system. The coating prepared after higher-temperature powder annealing (2 h @ 500 °C) in Fig. 4(b) shows a homogeneously covered coating surface. The higher annealing temperature of 500 °C leads to a lower moisture content and removal of possible other adsorbates. This results in better flowability and sprayability of the powder, which leads to a more homogenous coating. It furthermore allows for healing out microstructural defects, which results in improved photoelectrochemical performance (Ref 32). Interestingly, all the coatings processed with carrier gas flows within the range from 7 to 25 l/min showed a similar appearance. In order to ensure homogeneous surface coverage and reproducible testing conditions, only AD samples with prior powder heat treatments of 2 h at 500 °C were considered for further evaluation.
Coating Microstructures
Figure 5 shows the cross sections of BiVO4 processed by AD by varying the gas flow rate and the traverse line speed of the robot system to study general influences on coating formation. All three coatings appear as rather inhomogeneous with locally varying thickness. For these coatings, the mean coating thickness only slightly varies in a range from about 500 to 600 nm. The coating thickness here being significantly smaller than the mean powder size indicates that layer formation is associated with particle fracture. At the carrier gas flow of 10 l/min, increasing the line speed from 10 to 30 mm/s only slightly decreases the coating thickness from about 550 nm to about 520 nm. Such behavior is unexpected, since the lower particle coverage per surface unit at higher traverse speeds should scale with layer thickness. A possible explanation for the nonlinear behavior is given by secondary impacts of larger particles that cause erosion. In addition, the coating processed at the higher traverse speed seems to be more homogenous with lower amounts of voids, however showing some cracks at the interface to the underlying FTO layer. The higher porosity of the sample processed at the lower traverse speed of 10 mm/s might be attributed to increased influence of the following impacts causing partly fracture and erosion of previously adhering particles, here possibly being enhanced by comparatively larger sizes of the feedstock powder. At a traverse speed of 30 mm/s, increasing the gas flow rate results in a slightly higher coating thickness of about 590 nm and a lower porosity as compared to the previously described one. The darker contrast is caused by secondary phases being present in the coating. The powder feedstock contained vanadium oxide (most likely V2O5) only in small amounts, but these vanadium oxide particles seem to have no influence on the coating formation. As shown in Fig. 5, these vanadium oxide impurities are well integrated to the coating in a similar manner as the BiVO4 particles. V2O5 is present in the spray powder by decomposition during powder annealing, but also grows by coating annealing. Hence, it is difficult to distinguish individual contributions as shown in Fig. 6.
As an example, for the rather homogeneous AD coating processed with a gas flow of 10 l/min and a traverse speed of 10 mm/s, Fig. 7 shows the SEM micrographs of the surface topography and the cross-section microstructures. Despite the feedstock powder having a mean size of about 5 µm, topographical features on the coating surface (Fig. 5a) rarely exceed dimensions of 2 µm. On the surface, most prominently, features of fracture and fragmentation can be observed. Only to a minor extent, pattern of deformation craters or loosely bonded particles can be seen. The cross sections prepared by FIB of the SEM in Fig. 5(b) and (c) show the BiVO4 coating buildup on the FTO layer and the glass substrate as indicated in the micrographs. The BiVO4 coating shows a rather rough surface, with irregularities originating from loosely bonded particles on the surface and partly fractured ones. In addition, pores close to the surface are observed. In contrast, the lower part of the coating appears to be more homogeneous with less cracks and pores. According to the section shown in Fig. 7(b) and (c), a coating thickness of 150-400 nm was obtained. The details seen by STEM in Fig. 7(c) reveal that the obtained thin film is rather dense and nanostructured. Moreover, even under the high resolution of Fig. 7(c), no gaps or cracks are visible between the adhering BiVO4 coating and the FTO grains of the substrate layer. Particle–particle interfaces are well bonded and show almost no interstices. Brighter parts in these micrographs arise from FIB thinning procedures as they can be observed throughout the whole sample. The nearly white areas correspond to pores or very thin areas, not causing absorption contrast.
SEM micrographs showing microstructural details of an AD BiVO4 coating on FTO-coated glass substrates: (a) surface morphology, (b) cross section view prepared by FIB, (c) STEM lamella prepared by FIB. The coating was processed by AD using a gas flow rate of 10 l/min and a traverse line speed of 10 mm/s
Micrographs of the reference samples prepared by the CS method are shown in Fig. 8. The surface topography and a cross-sectional view of the cold-sprayed sample are presented in Fig. 8(a) and (b), respectively. The substrate seems to be fully covered, though coating thickness is non-uniform, varying between 1 and 8 µm. Small cracks and pores are present throughout the coating. Moreover, an interlayer is formed between the BiVO4 and the Ti substrate, most likely TiOx arising from reactions during the annealing procedure as shown for another area with a respective EDS line scan in Fig. 9.
Coating Cohesion
Polished Ti was used as a substrate for the initial investigations into AD coating cohesion for BiVO4. Cavitation testing was performed on AD samples for 5, 10 and 30 s in order to determine the topological changes of the coating surface under the externally applied loads. Figure 10(a) shows a BiVO4 layer on titanium substrate AD sprayed at 10 mm spraying distance and with a flow rate of 10 l/min N2 before the cavitation test (a) and after 30-s exposure against cavitation (b). For better comparison, the identical area on the specimen was investigated in both analyses. The sample in Fig. 10(a) shows a rather homogeneously covered surface with some height differences as distinguishable by the contrast. Some brighter parts also indicate loosely bound particles on the surface. After 30 s exposure to the cavitation test, the sample surface is still intact, preserving the topographical features of the as-sprayed sample. The major difference concerns the loosely bonded particles on the sample surface, which have vanished during cavitation testing. Since no significant amounts of the coating got removed as flakes, these results can be taken as a measure for rather good coating cohesion. Furthermore, despite the small thickness, no area could be detected showing the substrate surface.
Photoelectrochemical Performance
Photocurrent densities were measured in PEC cells in order to determine the water splitting performance as a measure of the functional properties of the different coatings. In order to improve the charge carrier transport by eliminating defects, which may act as recombination centers, all coated samples were annealed in air at 500 °C for 2 h. As an example, Fig. 11 shows the current densities obtained in the cyclic voltammetry experiment of an AD-processed BiVO4 coating with and without illumination.
Cyclic voltammetry measurements performed on electrodes obtained by CS as well as by AD with different coating process parameters, in as sprayed and after heat treatment, are shown in Fig. 12. For the determination of the photocurrent densities, the current density of the measurements without illumination was subtracted from the values found under illumination. The corresponding data for the different coatings are compared in Table 3. The black lines in Fig. 12 indicate the thermodynamic water splitting potential at 1.23 V for which the obtained photocurrent densities in the table are taken for quantitative comparison. Under illumination by simulated sunlight, the heat-treated AD-sprayed BiVO4 coatings on FTO glass substrates show significantly better performance than the untreated ones. The capacitive effect seen in Fig. 11 and 12(d) can be attributed to the highly structured and partially porous BiVO4 coating produced by AD and CS, which results in high surface area and correspondingly high double-layer capacitance.
Photocurrent densities from voltammetry measurements, as-sprayed (black) and after heat treatment in air for 2 h at 500 °C (blue), for the samples discussed in the text. (a), (b) and (c) AD coatings obtained on FTO glass with different process parameter sets (see text), illuminated from the back; (d) CS coating on Ti, illuminated from the front. All measurements under AM1.5G illumination, in pH7 buffer, with added hole scavenger (Na2SO3). The thermodynamic water splitting potential of 1.23 V vs. the reversible hydrogen electrode (V vs. RHE) is indicated with a vertical black line. Due to the clarity of the images, the dark current is not shown here
Discussion
Influence of Powder Morphology and Pre-treatment on AD
Regarding the results depicted in Fig. 4(a), the non-complete layer formation by using powder after low-temperature annealing is attributed to the inhomogeneously dispersed powder in the aerosol generation unit as a consequence of agglomerated powder particles. As compared to 200 °C, the higher annealing temperature of 500 °C of the powder prior to spraying leads to a homogeneously covered sample surface, as shown in Fig. 4(b). Adsorbed water evaporates from particle surfaces and reduces powder agglomeration, allowing for defined dispersion in the carrier gas stream. Therefore, the amount of sprayed powder is more controllable and continuous. It should be noted that powder agglomeration also leads to non-defined, large sizes that reach lower velocities in the aerosol jet and thus do not contribute to bonding and coating formation.
Coating Formation in AD
Comparing AD results from powder and coating analyses, morphological and microstructural features of the coatings are significantly smaller than those of the feedstock material. The observed refinement in principle could be attributed to two different factors: (i) Only smaller particles can bind to the substrate and contribute to aerosol coating formation and (ii) bigger particles fracture and only remnants remain bonded to the substrate. Molecular dynamics (MD) simulations stated (Ref 33) that under certain boundary conditions, plastic deformation of the ceramic particles as well as fracture occurs in AD. This theory is supported by the work of Calvié et al. (Ref 34) who show ceramic particles in the nano-size regime can plastically deform and by the work of Park et al. (Ref 35,36,37,38) who show a shock-induced plasticity and fragmentation phenomena for aerosol-sprayed alumina particles. Under certain boundary conditions, the latter could be followed by crack closure. The prominence of these features depends on the particle velocity and size. General dependencies can be represented in a window of deposition offering information about critical values and an optimal range to obtain bonding (Ref 39). For AD-sprayed BiVO4, it is currently under investigation, whether plastic deformation may take place, or which other mechanisms might contribute such as crack closure.
For some materials systems, velocities and size regimes for successful bonding in AD have been defined. One approach to investigate particle velocities experimentally was carried out by Lebedev et al. using a time-of-flight method to determine particle velocities (Ref 40). To cast more light onto the influence of spraying conditions on particle velocities, computational fluid dynamics (CFD) was applied (Ref 33). The following main results were derived: (i) The gas stream reaches supersonic velocity after exiting a sonic nozzle into a vacuum chamber, (ii) small particles can reach up to 75% of the gas stream velocity, (iii) longer spray distances are favorable due to lower bow-shock effect at the substrate surface, and (iv) a higher pressure difference between aerosol generation unit and deposition chamber leads to higher particle velocities. Following these results, longer spraying distances of 30-40 mm were considered in this work, in order to minimize the bow-shock effect. Additionally, the pressure in the aerosol generation unit was raised by choosing higher gas flow rates. Although critical velocities need to be taken into account for obtaining bonding in AD, it might be possible that bigger particles could erode already deposited layers.
Coating Integrity of AD Coatings
As shown in Fig. 10, only loosely bonded particles are removed after a cavitation time of 30 s. The basic surface topography of the coating is retained after cavitation testing, and also, no delamination of the coating can be observed. This signifies good cohesion and adhesion properties of the AD-sprayed coating, resisting the cyclic shock loads by imploding gas bubbles for at least 30 s in cavitation testing. Good cohesion and adhesion in terms of interface qualities are prerequisites for sufficiently high charge transfer in PEC cells and later applications to guarantee a mechanically stable material for long-time performances. The removal of the few single particles from the surface implies that these particles are not bonded to previous layers and thus do not contribute to coating formation. Interfaces of these particles to already deposited layers did not reach conditions to guarantee sufficient cohesion over common grain boundaries for intimate bonding. That could be attributed to non-sufficiently high impact velocities of these species.
Water Splitting Performance
The BiVO4 coatings on FTO/glass obtained by AD in this work show an average thickness of around 500 nm. Such a low coating thickness is essential for photoelectrochemical applications, in particular for materials with limited carrier transport properties, as is the case for BiVO4. Electron–hole pairs generated within the anode coating material by direct sunlight radiation have to be separated, after which holes are transported to the surface of the anode where they propel the oxygen evolution reaction, while electrons are transported to the back contact and further to the counter electrode to be used for hydrogen generation. This transport of charge carriers to opposing interfaces has to be fast, before they are annihilated by recombination. Thin films imply shorter diffusion distances and can thus enable higher photoefficiencies (Ref 41). This highlights one major advantage of AD over CS for this application, as the particle size limit in CS consistently results in thicker films.
Highly structured coatings can be produced with both AD and CS. A nanostructured morphology that is accessible for illumination is desirable as it enhances light absorption due to internal reflections. Additionally, the increased area of contact with the electrolyte enhances electrochemical reactivity (though it will also increase losses by surface recombination). A good adhesion at the interface between coating and substrate and cohesion within the coating are key factors to achieve faster diffusion of the electrons and holes and to minimize ohmic losses and bulk recombination. Furthermore, the coating has to be stable during oxygen production in aqueous solution, which requires a layer with structural integrity.
The significantly higher current densities observed in this work for annealed samples in comparison with as-sprayed ones can be attributed to healing of crystal defects and to a general improvement of the internal interface quality in the coatings. Typically, a photocurrent density enhancement of about eight- to tenfold was reached for thermally annealed samples.
The surface topography of all electrodes is comparable, with a surface roughness of about 2 µm (see “Coating Microstructures” section). This excludes a mayor impact of the surface topography on the obtained photocurrent densities (please see Table 3 for the measured photocurrent densities). FIB cross sections (Fig. 5), on the other hand, reveal differences between the microstructure of these samples: The three process parameter sets explored result in a different porosity and phase composition.
The highest current density is found for samples that originate from an AD process with a small gas flow rate and a slow traverse line speed (Fig. 12a). The BiVO4 layers formed under these conditions are rather porous, featuring larger cavities in the BiVO4 layer, while the coatings appear to be well attached to the FTO/glass substrate. In our measurements under back illumination, the relatively high performance of these samples can be attributed to a combination of adequate back contact and low effective absorber thickness.
The combination of a low gas flow rate and a fast traverse line speed results in the formation of dense layers (as shown in Fig. 5, center image) that do not appear to be as firmly attached to the substrate as it is seen for the other samples (Fig. 12b). When comparing the dark currents for all measurements, it is evident that the measured dark currents after annealing the sample are usually lower than in the as-sprayed state (compare Fig. 11). The behavior of this electrode, however, indicates an opposite direction, which counteracts partially the benefits of crystal structure healing. This may be attributed to the partial detachment of the film from the back contact, due to reorganization during annealing under internal stress. The resulting increase in back-contact resistance additionally causes an anodic shift in the onset potential for the photocurrent.
The second highest photocurrent density for the investigated electrodes is found for the third parameter set with the highest gas flow rate and the highest table speed (Fig. 12c). The FIB/SEM cross section shows a formation of a slightly porous layer with Bi-rich and V-rich phases in the BiVO4 layer that seems to be strongly attached to the FTO/glass substrate. The adequate performance, obtained despite the fact that phase separation took place during the annealing step, highlights the importance of the strong attachment of the layers to the substrate. Conditions and influences of the phase separation are currently under investigation in our laboratories.
Considering that the produced AD BiVO4 samples do not contain any dopants, their photoelectrochemical performances already show superior behavior for water oxidation and water splitting as compared to other low-cost coatings (Ref 42). Attainable performance of the undoped BiVO4 thin films could probably be further improved by designing thinner layers, with more homogeneous powder feed rates, and with smaller powder feedstock during AD by using non-agglomerated powder particles.
The photoelectrochemical behavior of a CS-coated sample is shown in Fig. 12(d) as reference. It should not be interpreted as a direct comparison of the performance obtainable with both coating technologies. On the one side, a different BiVO4 powder was used, due to the limitations in particle size imposed by the more pronounced bow-shock effect in CS. Furthermore, while the shown morphology of the CS coating can be considered as a representative example for the materials system considered, the performance could be further optimized by tuning process parameters and the resulting coating microstructure and thickness.
Nevertheless, it is useful to emphasize one significant advantage of AD over CS for PEC applications, which is the possibility of coating more delicate substrates, such as FTO-coated glass. This enables the construction of stacked devices, able to capture additional light. It furthermore enables backside illumination. This is particularly relevant for BiVO4, in which electron transport is performance limiting, which leads to increased recombination losses when light reaches the coatings from the front (Ref 21, 41).
A further significant advantage of AD for PEC application, related to the use of smaller particles, is the lower limit coating thickness that is achievable. Finally, the use of metallic substrates, as in the case of the CS sample presented here, limits the range of post-deposition thermal treatments. Contrary to the behavior seen for all AD samples coated on FTO glass, for the CS sample shown here, the photocurrent decreases after heat treatment. This can be attributed to the growth of a TiOx layer at the interface between coating and substrate, which results in an increased back-contact resistance, with corresponding significant ohmic losses.
Summary and Conclusions
BiVO4 coatings were successfully deposited on FTO-coated glass and titanium substrates using the aerosol deposition (AD) method. In this preliminary study, three sets of AD deposition parameters were explored, by tuning process gas flow and traverse line speed.
For the deposition conditions investigated, the resulting film present thickness around 500 nm and in general good internal coating integrity and good bonding to the substrate. Dual-beam SEM/FIB and STEM micrographs reveal a considerable impact of the process parameters on the coating microstructure, with lower traverse line speeds resulting in porous films. Cyclic voltammetry measurements under simulated sunlight show a significant influence of the coating microstructure on the photoelectrochemical performance, which can be further enhanced by post-deposition thermal treatment.
AD presents major advantages over cold spraying (CS) for the fabrication of water splitting photoelectrodes. The use of smaller particles in AD results in sub-micrometer thick coatings, which is necessary for the implementation of promising light absorbing materials with poor charge transport properties such as BiVO4. Furthermore, the lower kinetic energy of the feedstock powder enables the use of delicate substrates, which greatly expands the range of attainable device configurations.
References
S.D. Tilley, S. Lany, and R.V.D. Krol, Advances in Photoelectrochemical Water Splitting: Theory, Experiment and Systems Analysis, Vol 290, 1st ed., Royal Society of Chemistry, London, 2018
D. Gielen, F. Boshell, D. Saygin, M.D. Bazilian, N. Wagner, and R. Gorini, The Role of Renewable Energy in the Global Energy Transformation, Energy Strategy Rev., 2019, 24, p 38-50
M. Klell, H. Eichlseder, A. Trattner, Wasserstoff in der Fahrzeugtechnik—Erzeugung, Speicherung, Anwendung, Springer Fachmedien Wiesbaden GmbH, 2018
S. Sengodan, R. Lan, J. Humphreys, D. Du, W. Xu, H. Wang, and S. Tao, Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications, Renew. Sustain. Energy Rev., 2018, 82, p 761-780
J. Bellosta von Colbe, J.-R. Ares, J. Barale, M. Baricco, C. Buckley, G. Capurso, N. Gallandat, D.M. Grant, M.N. Guzik, I. Jacob, E.H. Jensen, T. Jensen, J. Jepsen, T. Klassen, M.V. Lototskyy, K. Manickam, A. Montone, J. Puszkiel, S. Sartori, D.A. Sheppard, A. Stuart, G. Walker, C.J. Webb, H. Yang, V. Yartys, A. Züttel, and M. Dornheim, Application of Hydrides in Hydrogen Storage and Compression: Achievements, Outlook and Perspectives, Int. J. Hydrogen Energy, 2019, 44(15), p 7780-7808
W.-H. Cheng, M.H. Richter, M.M. May, J. Ohlmann, D. Lackner, F. Dimroth, T. Hannappel, H.A. Atwater, and H.-J. Lewerenz, Monolithic Photoelectrochemical Device for Direct Water Splitting with 19% Efficiency, ACS Energy Lett., 2018, 3(8), p 1795-1800
Z. Li, W. Luo, M. Zhang, J. Feng, and Z. Zou, Photoelectrochemical Cells for Solar Hydrogen Production: Current State of Promising Photoelectrodes, Methods to Improve Their Properties, and Outlook, Energy Environ. Sci., 2013, 6(2), p 347-370
Z. Zou, J. Ye, K. Sayama, and H. Arakawa, Direct Splitting of Water Under Visible Light Irradiation with an Oxide Semiconductor Photocatalyst, Nature, 2001, 414(6864), p 625-627
Z.-F. Huang, L. Pan, J.-J. Zou, X. Zhang, and L. Wang, Nanostructured Bismuth Vanadate-Based Materials for Solar-Energy-Driven Water Oxidation: A Review on Recent Progress, Nanoscale, 2014, 6(23), p 14044-14063
F.M. Toma, J.K. Cooper, V. Kunzelmann, M.T. McDowell, J. Yu, D.M. Larson, N.J. Borys, C. Abelyan, J.W. Beeman, K.M. Yu, J. Yang, L. Chen, M.R. Shaner, J. Spurgeon, F.A. Houle, K.A. Persson, and I.D. Sharp, Mechanistic Insights into Chemical and Photochemical Transformations of Bismuth Vanadate Photoanodes, Nat. Commun., 2016, 7, p 12012
B.-C. Xiao, L.-Y. Lin, J.-Y. Hong, H.-S. Lin, and Y.-T. Song, Synthesis of a Monoclinic BiVO4 Nanorod Array as the Photocatalyst for Efficient Photoelectrochemical Water Oxidation, RSC Adv., 2017, 7(13), p 7547-7554
J. Akedo and M. Lebedev, Microstructure and Electrical Properties of Lead Zirconate Titanate (Pb(Zr52/Ti48)O3) Thick Films Deposited by Aerosol Deposition Method, Jpn. J. Appl. Phys., 1999, 38(Part 1, No. 9B), p 5397-5401
J. Akedo, Aerosol Deposition Method for Fabrication of Nano Crystal Ceramic Layer, Mater. Sci. Forum, 2004, 449–452, p 43-48
J. Akedo, Room Temperature Impact Consolidation (RTIC) of Fine Ceramic Powder by Aerosol Deposition Method and Applications to Microdevices, J. Therm. Spray Technol., 2008, 17(2), p 181-198
H.J. Kim, Y.J. Yoon, J.H. Kim, and S.M. Nam, Application of Al2O3-Based Polyimide Composite Thick Films to Integrated Substrates Using Aerosol Deposition Method, Mater. Sci. Eng., B, 2009, 161(1), p 104-108
J. Akedo, S. Nakano, J. Park, S. Baba, and K. Ashida, The Aerosol Deposition Method—For Production of High Performance Micro Devices with Low Cost and Low Energy Consumption, Synthesiol. Engl. Ed., 2008, 1(2), p 121-130
Y. Hasegawa, Y. Sato, and S. Yoshikado, Fabrication of Tin-Doped Indium Oxide Thin Films Using Aerosol Deposition, J. Ceram. Sci. Technol., 2016, 7(4), p 429-432
A. Vardelle, C. Moreau, J. Akedo, H. Ashrafizadeh, C.C. Berndt, J.O. Berghaus, M. Boulos, J. Brogan, A.C. Bourtsalas, A. Dolatabadi, M. Dorfman, T.J. Eden, P. Fauchais, G. Fisher, F. Gaertner, M. Gindrat, R. Henne, M. Hyland, E. Irissou, E.H. Jordan, K.A. Khor, A. Killinger, Y.-C. Lau, C.-J. Li, L. Li, J. Longtin, N. Markocsan, P.J. Masset, J. Matejicek, G. Mauer, A. McDonald, J. Mostaghimi, S. Sampath, G. Schiller, K. Shinoda, M.F. Smith, A.A. Syed, N.J. Themelis, F.-L. Toma, J.P. Trelles, R. Vassen, and P. Vuoristo, The 2016 Thermal Spray Roadmap, J. Therm. Spray Technol., 2016, 25(8), p 1376-1440
H. Ye, J. Lee, J.S. Jang, and A.J. Bard, Rapid Screening of BiVO4-Based Photocatalysts by Scanning Electrochemical Microscopy (SECM) and Studies of Their Photoelectrochemical Properties, J. Phys. Chem. C, 2010, 114(31), p 13322-13328
F.M. Toma, J.K. Cooper, V. Kunzelmann, M.T. McDowell, J. Yu, D.M. Larson, N.J. Borys, C. Abelyan, J.W. Beeman, K.M. Yu, J. Yang, L. Chen, M.R. Shaner, J. Spurgeon, F.A. Houle, K.A. Persson, and I.D. Sharp, Mechanistic Insights into Chemical and Photochemical Transformations of Bismuth Vanadate Photoanodes, Nat. Commun., 2016, 7(1), p 12012
Y. Liang, T. Tsubota, L.P.A. Mooij, and R. van de Krol, Highly Improved Quantum Efficiencies for Thin Film BiVO4 Photoanodes, J. Phys. Chem. C, 2011, 115(35), p 17594-17598
T. Stoltenhoff, H. Kreye, and H.J. Richter, An Analysis of the Cold Spray Process and Its Coatings, J. Therm. Spray Technol., 2002, 11(4), p 542-550
T. Schmidt, H. Assadi, F. Gärtner, H. Richter, T. Stoltenhoff, H. Kreye, and T. Klassen, From Particle Acceleration to Impact and Bonding in Cold Spraying, J. Therm. Spray Technol., 2009, 18(5), p 794
T. Emmler, H. Gutzmann, P. Hillebrand, M. Schieda, R. Just, F. Gärtner, P. Bogdanoff, I. Herrmann-Geppert, and T. Klassen, Cold Gas Spraying of Semiconductor Coatings for the Photooxidation of Water, Solar Hydrogen Nanotechnol., 2013, 8822, p 88220C-88212
I. Herrmann-Geppert, P. Bogdanoff, H. Gutzmann, T. Dittrich, T. Emmler, R. Just, M. Schieda, F. Gärtner, and T. Klassen, Cold Gas Sprayed TiO2-Based Electrodes for the Photo-Induced Water Oxidation, ECS Trans., 2014, 58(30), p 21-30
H. Gutzmann, Kaltgasspritzen von Titandioxid—Bindungsverhalten und Photokatalytische Eigenschaften, Helmut-Schmidt-University Hamburg, Hamburg, 2014
J.O. Kliemann, H. Gutzmann, F. Gärtner, H. Hübner, C. Borchers, and T. Klassen, Formation of Cold-Sprayed Ceramic Titanium Dioxide Layers on Metal Surfaces, J. Therm. Spray Technol., 2011, 20(1–2), p 292-298 ((in English))
I. Herrmann-Geppert, P. Bogdanoff, T. Emmler, T. Dittrich, J. Radnik, T. Klassen, H. Gutzmann, and M. Schieda, Cold Gas Spraying—A Promising Technique for Photoelectrodes: The Example TiO2, Catal. Today, 2016, 260, p 140-147
I. Ivanova, J. Schneider, H. Gutzmann, J.-O. Kliemann, F. Gärtner, T. Klassen, D. Bahnemann, and C.B. Mendive, Photocatalytic Degradation of Oxalic and Dichloroacetic Acid on TiO2 Coated Metal Substrates, Catal. Today, 2013, 209, p 84-90
D. Hanft, J. Exner, M. Schubert, T. Stöcker, P. Fuierer, and R. Moos, An Overview of the Aerosol Deposition Method: Process Fundamentals and New Trends in Materials Applications, J. Ceram. Sci. Technol., 2015, 6(3), p 147-182
J.-H. Park, D.-S. Park, B.-D. Hahn, J.-J. Choi, J. Ryu, S.-Y. Choi, J. Kim, W.-H. Yoon, and C. Park, Effect of Raw Powder Particle Size on Microstructure and Light Transmittance of α-Alumina Films Deposited by Granule Spray in Vacuum, Ceram. Int., 2016, 42(2), p 3584-3590
L. Shi et al., Annealing Temperature Effects on Photoelectrochemical Performance of Bismuth Vanadate Thin Film Photoelectrodes, RSC Adv., 2018, 8, p 29179-29188
C. Wolpert, M.V. Vidaller, H.J. Richter, B. Daneshian, M. Schieda, F. Gärtner, T. Klassen, Aerosol Spraying of Nanoparticles for Hydrogen Technology, 11. Kolloquium Hochgeschwindigkeits-Flammspritzen/HVOF Spraying, in Gemeinschaft Thermisches Spritzen e.V., ed. by C. Penszior, W. Krömmer, (Association of Thermal Sprayers, 2018) (Erding), c/o Linde AG, pp. 31–39
E. Calvié, J. Réthoré, L. Joly-Pottuz, S. Meille, J. Chevalier, V. Garnier, Y. Jorand, C. Esnouf, T. Epicier, J. Quirk, and K. Masenelli-Varlot, Mechanical Behavior Law of Ceramic Nanoparticles from Transmission Electron Microscopy In Situ Nano-compression Tests, Mater. Lett., 2014, 119, p 107-110
H. Park, J. Kwon, I. Lee, and C. Lee, Shock-Induced Plasticity and Fragmentation Phenomena During Alumina Deposition in the Vaccum Kinetic Spraying Process, Scripta Mater., 2015, 100, p 44-47
H. Park, J. Kim, and C. Lee, Dynamic Fragmentation Process and Fragment Microstructure Evolution of Alumina Particles in a Vacuum Kinetic Spraying System, Scripta Mater., 2015, 108, p 72-75
H. Park, J. Kim, S.B. Lee, and C. Lee, Correlation of Fracture Mode Transition of Ceramic Particle with Critical Velocity for Successful Deposition in Vacuum Kinetic Spraying Process, J. Therm. Spray Technol., 2017, 26, p 327-339
H. Kwon, Y. Kim, H. Park, and C. Lee, Impact Behavior for Successful Particle–Particle Bonding in Vacuum Kinetic Spraying, J. Therm. Spray Technol., 2020, https://doi.org/10.1007/s11666-020-01078-7
B. Daneshian and H. Assadi, Impact Behavior of Intrinsically Brittle Nanoparticles: A Molecular Dynamics Perspective, J. Therm. Spray Technol., 2014, 23(3), p 541-550
M. Lebedev, J. Akedo, K. Mori, and T. Eiju, Simple Self-Selective Method of Velocity Measurement for Particles in Impact-Based Deposition, J. Vac. Sci. Technol., A, 2000, 18(2), p 563-566
F.F. Abdi, T.J. Savenije, M.M. May, B. Dam, and R. van de Krol, The Origin of Slow Carrier Transport in BiVO4 Thin Film Photoanodes: A Time-Resolved Microwave Conductivity Study, J. Phys. Chem. Lett., 2013, 4(16), p 2752-2757
Y. Ma, S.R. Pendlebury, A. Reynal, F. Le Formal, and J.R. Durrant, Dynamics of Photogenerated Holes in Undoped BiVO4 Photoanodes for Solar Water Oxidation, Chem. Sci., 2014, 5(8), p 2964-2973
Acknowledgments
The authors thank the team of the HSU and HZG, particularly Henning Gutzmann for the preparation of the cold-sprayed reference samples, as well as the team of Dr. Akedo at AIST for experimental support. The authors gratefully acknowledge financial support by the Federal Ministry of Education and Research of Germany, in the framework of the Project “FocusH2” (No. 03SF0479A). Furthermore, the authors thank Prof. H. Richter for information on fluid dynamic results and for insightful discussions.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a special topical focus in the Journal of Thermal Spray Technology on Aerosol Deposition and Kinetic Spray Processes. This issue was organized by Dr. Kentaro Shinoda, National Institute of Advanced Industrial Science and Technology (AIST); Dr. Frank Gaertner, Helmut-Schmidt University; Prof. Changhee Lee, Hanyang University; Prof. Ali Dolatabadi, Concordia University; and Dr. Scooter Johnson, Naval Research Laboratory.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolpert, C., Emmler, T., Villa Vidaller, M. et al. Aerosol-Deposited BiVO4 Photoelectrodes for Hydrogen Generation. J Therm Spray Tech 30, 603–616 (2021). https://doi.org/10.1007/s11666-020-01104-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-020-01104-8