Abstract
In this work, tungsten oxide (WO3) films were deposited at room temperature and annealed for 2 h at 400°C. The electrochromic and electrochemical properties were studied for two different electrolytes. The films were deposited at different oxygen flow rates of 2, 4, and 6 standard cubic centimeters per minute (SCCM). X-ray diffraction analysis revealed structural characterization of amorphous and crystalline phases. UV-visible spectroscopy optical transmittance revealed 91% transmittance, and energy-dispersive x-ray spectroscopy (EDS) analysis revealed the absence of impurities and the presence of W and O. An electrochemical analyzer was used to characterize the deposited and annealed WO3 films immersed in the two different electrolyte solutions (H2SO4 and LiClO4 with oxygen flow rates ranging from 2 SCCM to 6 SCCM). It was found that the H2SO4 electrolyte of an annealed WO3 thin film at 2 SCCM demonstrated high coloring efficiency of 50.18 cm2/C, and the LiClO4 electrolyte of an annealed WO3 thin film at 4 SCCM demonstrated high coloring efficiency of 20.06 cm2/C.
Similar content being viewed by others
Introduction
With the continuing rapid depletion of fossil fuel materials, renewable energy sources are increasingly used for electrochemical capacitors.1,2 Up to 30–40% of the primary energy consumed worldwide is utilized for heating, cooling, ventilation, and electrical appliances in buildings.3,4,5 Therefore, the use of materials in buildings that can convert and store energy from renewable sources is beneficial. By applying a small electric field, electrochromic substances can change their optical characteristics such as transmittance, reflection, and absorption in a reversible manner. This property makes them extremely appealing for a variety of potential applications, including large-area information displays, antiglare automobile mirrors, and energy-efficient smart windows.6,7
Numerous studies have been conducted on tungsten trioxide (WO3), which has been named one of the most interesting inorganic electrochromic compounds.8,9 An electrochromic material should have 100% optical modulation, meaning that it should be totally opaque while colored and fully transparent when bleached. The coloration efficiency (CE) and kinetics will depend on the morphological, structural, and compositional characteristics, and the suitability for use in a variety of devices, including solar cells,10,11 smart windows,12,13,14,15 gas sensors,16 and photodetectors.17 The electrochemical equation below18 controls the electrochromism reaction in WO3:
where x is the ion concentration, and M represents K+, H+, Na+, or Li+ ions. Various deposition techniques have been used for WO3 films including sol–gel,19 hot-filament-assisted synthesis,20 sputtering,21,22,23,24,25,26,27,28,29,30,31,32,33 electron beam evaporation,34,35 electrodeposition processing,36 and hydrothermal37,38,39,40,41 and solvothermal techniques.42 Overall, relatively little research on sputtering-fabricated WO3 films has been published. Furthermore, up to this point, we were unable to locate any other research in the literature demonstrating the influence of the deposition oxygen flow rate (OFR) on the electrochromic properties of WO3 films produced by sputtering. Therefore, we present a thorough investigation of the effects of various oxygen flow deposition rates, post-annealed WO3 films, and various electrolyte solutions on the optical, structural, electrochromic, and electrochemical attributes of WO3 films deposited by the sputtering technique in the current work. Here, we discuss how various OFRs affect the structural, optical, and electrochromic characteristics of sputtering. WO3 films were produced, and efforts were made to optimize the OFR, the electrolyte solution, and the nature of the film (amorphous or crystalline) for use in electrochromic applications.
Experimental Techniques
Method to Produce Thin Films of WO3
A popular physical vapor deposition (PVD) technique known as direct current (DC) magnetron sputtering was used to deposit the WO3 films on Corning glass and fluorine-doped tin oxide (FTO) substrates. Before being fed into the sputtering chamber, the glass samples were cleaned with a soap solution and deionized water (DI) using ultrasonication. The tungsten metal disc was used as the sputter target and it was sputtered in an atmosphere of argon (Ar) and oxygen (O2). The base vacuum of the sputtering chamber was maintained at 2 × 10−6 mbar. In order to remove impurities that had been adsorbed onto the target surface pre-sputter, the tungsten target was subjected to an argon gas atmosphere for 10 min. The WO3 films were produced under OFRs of 2, 4, and 6 standard cubic centimeters per minute (SCCM) at room temperature (RT). A constant 100 mA DC current was used during the whole deposition.
Characterization
X-ray diffraction (XRD; Rigaku MiniFlex, Rigaku Corporation) was used to analyze the crystallization of the films. Raman spectroscopy (Renishaw plc) was used to analyze the chemical bonding. The optical absorption behavior was characterized using an ultraviolet–visible spectrometer (SPECORD S600). An electrochemical workstation (SP-300 BioLogic) was used for all electrochemical analyses. The workstation has a three-electrode setup with a platinum wire as the counter electrode (CE), Hg2/HgCl2 as the reference electrode (RE), and WO3 thin film as the working electrode (WE). An aqueous solution of 0.5 M H2SO4 and a non-aqueous solution of 0.5 M LiClO4 were used as electrolytes, and the sweep voltage was maintained between −0.7 and +1 V at scan rates of 20 mV/s.
Results and Discussion
XRD Analysis
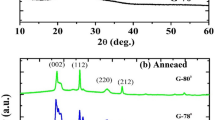
Figure 1a displays the XRD characteristics of the WO3 films that were deposited using DC sputtering at various OFRs. The RT-deposited samples exhibit amorphous characteristics at various OFRs, according to the XRD evaluation. Amorphous films are produced on a glass substrate that has not been heated when different amounts of PaO2 are deposited using DC and RF sputtering, according to studies by Madhavi et al.43 and Mohamed et al.44
An eventual change in film crystallinity is the most fundamental effect of the annealing process. Consequently, XRD analysis was used to characterize the WO3 films annealed at 400°C for 2 h in order to investigate the change in the crystal structure. The XRD patterns of heat-treated WO3 films after 2 h at 400°C are clearly shown in Fig. 1b, which reveals the presence of distinctive diffraction peaks corresponding to the WO3 planes. For different oxygen partial pressures, the XRD spectra that are produced show that the 400°C air-annealed sample is crystalline.25 DC-sputtered WO3 films have 2θ values of 14.04°, 23.38°, 24.47°, 28.16°, 33.89°, 36.93°, 50.09°, and 56.09°, and their hkl values are (100), (002), (020), (112), (220), (222), (002), and (102), respectively. There were differences in OFRs between 2 and 6 SCCM. For increased OFRs, WO3 films have a crystalline character, due to the annealing at 400°C.
Raman Analysis
The Raman spectra of the WO3 films at various OFRs are shown in Fig. 2a and b. Because of the W–O bonds, the spectra of the as-deposited film exhibit a significant peak at 770 cm−1, as shown in Fig. 3a.45 The appearance of this peak corresponds with the two strongest peaks at 719 and 807 cm−1 in the Raman spectrum of the crystalline WO3 films. The W=O stretching mode of terminal oxygen atoms is attributed to a rather steep peak at 950 cm−1, which may occur on the surfaces of the cluster and micro-void structures in the film.9 A weaker, broad peak is also present at 220 cm−1, which is attributed to the presence of W4+ states. Figure 2b displays the Raman spectra that were recorded for the WO3 annealed films. The spectra of WO3 films annealed at 400°C for 2 h at various OFRs exhibit a broad peak in the range of 100–1200 cm−1 due to the O–W–O stretching vibration mode, which denotes the growth of sub-stoichiometric WO3 films of mixed metallic tungsten and tungsten oxide. Sharp Raman peaks, representing the bending and stretching vibration modes of the O–W–O bond, can be seen at 695 and 809 cm−146 when the OFR increases from 2 to 6 SCCM.
Optical Properties
Figure 3 illustrates the transmittance plots of WO3 films for both RT and post-annealing at 400°C for 2 h at different OFRs. The transmittance is increased with respect to the increase in OFR for RT-deposited samples, and transmittance is decreased for annealed samples.47,48 The transmittance is more than 82% at a wavelength of 700 nm for RT samples at different OFRs, and transmittance is less for the sample annealed at 4 SCCM. The transmittance values for annealed and RT samples are shown in Table I.
Electrochemical Properties
The electrochemical properties determine the structure of the electrochromic WO3 thin film, which is employed as the working electrode. Hg/HgCl serves as the reference electrode, and a Pt needle serves as the counter electrode. For the half cell, 0.5 M H2SO4 and LiClO4 solution were prepared as electrolytes. The cyclic voltammetry (CV) curves of WO3 films were recorded in the potential range of −0.7 to 1 V at a scan rate of 20 mV s−1. When a negative voltage is supplied to the working electrode, the color changes from transparent to blue. When a positive voltage is applied, the film becomes transparent.49
Figures 4a and b and 6a and b show typical broad featureless peaks in the CV characteristics that represent the insertion and extraction of H+ and Li+ ions into WO3 films. The cathodic peak current of WO3 films at RT in the H2SO4 electrolyte solution, as measured by the CV curves (measured at −0.7 V), initially decreased from −2.98 mA/cm2 at 2 SCCM to −5.40 mA/cm2 at 6 SCCM OFR. This increase in OFRs eventually led to an increase in cathodic peak current as shown in Fig. 4a. The cathodic peak current of WO3 films at RT in LiClO4 electrolyte solution is shown in Fig. 4b. The CV curves initially decreased from −0.83 mA/cm2 at 2 SCCM to −1.42 mA/cm2 at 4 SCCM. This increase in OFRs ultimately led to an increase in cathodic peak current back to −0.74 mA/cm2 at 6 SCCM OFRs (Fig. 5). Figure 6 shows the first and 50th cyclic voltammetry curves of WO3 film deposited at RT, which reveal that the performance of the thin film was severely degraded and the stability decreased after 50 cycles. The reversibility decreased slightly as the number of cycles increased. The film contained certain irreversible deep trap sites, which prevented the electrochromic reversibility from reaching 100%. The area of the voltammogram decreased with an increase in the electrochemical cycle. An increase in voltage was observed with a decrease in current. The charge insertion capability of the film also decreased with increased cycles.50 The cathodic peak current of WO3 films in the H2SO4 electrolyte solution that was annealed at 400°C for 2 h showed a CV curve that initially decreased from −0.74 mA/cm2 at 2 SCCM to −0.92 mA/cm2 at 4 SCCM, as shown in Fig. 7a. However, as OFRs increased, the cathodic peak current eventually increased back to −0.87 mA/cm2 at 6 SCCM OFRs. The lowest cathodic peak current of all the CV curves is shown in Fig. 7b, which represents the cathodic peak current of annealed at 400°C in 2 h of WO3 films in LiClO4 electrolyte solution. The CV curves are recorded as −0.55 mA/cm2 at 2 SCCM, −0.48 mA/cm2 at 4 SCCM, and −0.39 mA/cm2 at 6 SCCM OFRs. Figures 5 and 8 show the comparison plots of CV curves of WO3 films RT and annealed for OFRs of 2 SCCM, 4 SCCM, and 6 SCCM. The Randles–Ševčík equation can be used to calculate the diffusion coefficient, D, for the H+ and Li+ ions.51
where ip, n, A, Co, v, and D are peak current, number of electrons, area of the active WO3 film, electrolyte concentration, scan rate, and diffusion coefficient, respectively.
Table II summarizes the determined diffusion coefficients of the WO3 films and annealed 400°C in 2 h of the WO3 films in both H2SO4 and LiClO4 electrolyte solutions treated at increasing OFRs. It is obvious that the diffusion coefficient (DC) is influenced by the OFRs and different electrolytes. Amorphous WO3 films in H2SO4 electrolyte treated at 2 SCCM OFR demonstrated a maximum diffusivity of 1.007 × 10−7 cm2/s, which is two orders of magnitude higher than the 1.59 × 10−9 cm2/s of the amorphous WO3 films in LiClO4electrolyte at 6 SCCM OFR. Additionally, crystalline WO3 films in H2SO4 electrolyte treated at 4 SCCM OFR showed the highest diffusivity of 2.32 × 10−9 cm2/s, which is an order of magnitude higher than the 4.53 × 10−10 cm2/s of the crystalline WO3 films in LiClO4 electrolyte at 6 SCCM OFR. These films were post-annealed at 400°C for 2 h. Finally, amorphous WO3 films in an electrolyte of H2SO4 at a 2 SCCM OFR revealed the highest DC of 1.007 × 10−7 cm2/s in comparison to crystalline WO3 films in an electrolyte of LiClO4 at 6 SCCM OFR, as shown in Fig. 9. Similar findings were reported by Madhavi et al., who found that the blockage of insertion sites was the reason for the decrease in DC at higher annealing temperatures.52 Hsu et al. explained that although there is a driving force from the applied voltage, the compact film structure prevented Li+ ions from intercalating in MoO3 electrochromic films that were produced using a sol–gel technique, which reduced the DC.53
Electrochromic Studies
Figures 10 and 11 show the optical transmission spectra of the WO3 films in their corresponding colored and bleaching states in the 300–900 nm range. The optical transmittance spectra of the DC-sputtered WO3 films were investigated by UV-visible spectroscopy. Figure 10a, b, c, d, and e displays the transmittance for the bleached and colored states obtained for various OFRs (2 SCCM, 4 SCCM, and 6 SCCM) and two different electrolyte solutions (H2SO4 and LiClO4). As shown in Fig. 11a, b, and c, the observed values of transmittance at a wavelength of 600 nm range from 0% for 2 SCCM, 1% for 4 SCCM, 5% for 6 SCCM for the colored state, and 58% for 2 SCCM, 85% for 2 SCCM, and 91% for 6 SCCM for the bleached state of WO3 films in 0.5 M H2SO4 electrolyte solution. In the colored state of the WO3 films in the 0.5 M LiClO4 electrolyte solution, the observed values of transmittance at a wavelength of 600 nm range from 19% for 2 SCCM, 10% for 4 SCCM, and 22% for 6 SCCM; and in the bleached state, the values are 43% for 2 SCCM, 81% for 2 SCCM, and 90% for 6 SCCM, as shown in Fig. 10d and e. Also, as measured for different OFRs (2 SCCM, 4 SCCM, and 6 SCCM) and two different electrolyte solutions (H2SO4 and LiClO4), the transmittance of annealed WO3 films (crystalline) for bleached and colored states is shown in Fig. 11 a, b, c, d, and e. As shown in Fig. 11a, b, and c, the observed values of transmittance at a wavelength of 600 nm range from 9% for 2 SCCM, 8% for 4 SCCM, and 15% for 6 SCCM for the colored state, and 66% for 6 SCCM for the bleached state of crystalline WO3 films in 0.5 M H2SO4 electrolyte solution. Figure 11d, e, and f shows the observed values of transmittance at a wavelength of 600 nm for crystalline WO3 films in 0.5 M LiClO4 electrolyte solution. The observed values range from 42% for 2 SCCM, 41% for 4 SCCM, and 64% for 6 SCCM for the colored state, and 68% for 2 SCCM, 60% for 2 SCCM, and 70% for 6 SCCM bleached state. We may deduce from this result that if OFRs in both electrolytes are increased, the films become less intensely colored because fewer ions are available for intercalation, making them less opaque and increasing the transmittance for the colored state.
The transmittance of these transition metal oxides in both transparent and opaque phases, which is necessary for electrochromic applications, is one of their most important properties. As a result, there is a significant optical modulation, or ΔOT, which is measured as the difference between the transmittance of the colored and bleached states.54 The host matrix’s trapped ions play a part in the material’s degraded electrochromic performance. Because the intercalated H+ ions are not entirely removed from the formed film, degradation in optical modulation of the host matrix can be detected during specific voltage cycles. CV measurements were performed, and the bleached and colored switching characteristics of WO3 and post-annealed WO3 films in H2SO4 and LiClO4 in two different electrolyte solutions of 0.5 M concentration were recorded at a scan rate of 10 mV/s and applied potential of −0.7 to 1 V. The results are shown in Table III for the various OFRs, as well as the WO3 and post-annealed WO3 films. The optical modulation of amorphous WO3 films in H2SO4 electrolyte solution is greatest at 6 SCCM OFR. However, WO3 films that were annealed at 400°C displayed very low bleaching and coloring transmittances, which is indicative of weak electrochromic characteristics. The crystalline WO3 films in LiClO4 electrolyte were unable to recover to their initial transparent form despite having poor transmittance in the colored state. Figure 12a and b shows the bleached and colored states obtained during oxidation and reduction, respectively. The coloring and bleaching times of the electrochromic films are plotted using chronoamperometry in Fig. 10. We fixed the pulse interval to 10 s, the start voltage to −0.7 V, and the upper limit voltage to 1 V for the chronoamperometry (CA) curve experiment using H2SO4 as the electrolyte. The response period for oxidation and reduction is the amount of time required for the anodic and cathodic currents to equalize when voltages are applied. The coloration time for 2 SCCM, 4 SCCM, and 6 SCCM of WO3 samples is 3.2, 3.7, and 3.3 s, respectively, and the bleach time for 2 SCCM, 4 SCCM, and 6 SCCM of WO3 samples is 1.7, 2.8, and 1.3 s, respectively. The outcome demonstrates that all deposited films took longer to color than to bleach (Fig. 13).
The ratio of the change in optical density to the incorporated charge per unit area is known as the coloring efficiency (CE), and it is one of the most important parameters for assessing the effectiveness of electrochromic products. It can be calculated using the formula shown below55:
where Tb is the bleached transmittance, Tc is the color transmittance, Qin is the incorporated charge, and ΔOD is the optical density. Using Tb and Tc from Table III, the CE of the WO3 films at 600 nm was then calculated as follows. For different OFRs, the calculated CE of the WO3 films (amorphous) was found to be 22 cm2/C at 2 SCCM, 21.16 cm2/C at 4 SCCM, 20.54 cm2/C at 6 SCCM in H2SO4 electrolyte solution, and 19.10 cm2/C at 2 SCCM, 27.44 cm2/C at 4 SCCM, and 18.46 cm2/C at 6 SCCM in LiClO4 electrolyte solution, respectively. The calculated CE of the heated WO3 films (crystalline) at 400°C for 2 h was found to be 50.18 cm2/C at 2 SCCM, 16.64 cm2/C at 4 SCCM, and 33.75 cm2/C at 6 SCCM in H2SO4 electrolyte solution, and 20.06 cm2/C at 2 SCCM, 18.25 cm2/C at 4SCCM, and 4.92 cm2/C at 6 SCCM in LiClO4 electrolyte solution, respectively. According to CE results, crystalline WO3 films in an electrolyte concentration of H2SO4 performed better in an electrochemical cell than amorphous WO3 films in an electrolyte concentration of LiClO4, as shown in Fig. 14.
In this study, we propose the coexistence of amorphous and crystalline phases in WO3 films annealed at 400°C at various OFRs (2, 4, and 6 SCCM) in two different electrolyte (H2SO4 and LiClO4) solutions. Since they demonstrated a good balance between ion storage capacity, optical modulation, and CE, they are recognized as the optical electrochromic features for electrochromic device applications. A higher CE was seen in the WO3 that was annealed at 400°C with a lower OFR (2 SCCM) and a higher concentration of the H2SO4 electrolyte solution but at the expense of switching properties, as shown by the prior data. The quick switching properties of crystalline WO3 films, however, occur at the expense of low optical modulation.
Conclusion
This study revealed the effects of various OFRs (2, 4, and 6 SCCM) and annealing temperature on the structural, electrochromic, and optical characteristics of DC magnetron-sputtered WO3 films. The WO3 films are amorphous, according to XRD measurements; however, they exhibit crystalline characteristics at 400°C after annealing. SEM scans of WO3 films revealed a uniform and smooth surface, which is a sign of its amorphous nature. The elimination of additional organic residues caused the WO3 films to become denser with more evident cracks. Energy-dispersive x-ray spectroscopy (EDS) investigation demonstrated the absence of contaminants in the grown film and provided confirmation that W and O were present in the deposited film. We observed and carefully examined the effects of changing OFR and two different electrolyte solutions (H2SO4 and LiClO4) on the electrochromic characteristics of the WO3 and post-annealed WO3 films. UV-visible spectrometry showed that the optical transmittance of higher OFRs and amorphous WO3 samples was greater than that of lower OFRs and air-annealed samples. The amorphous WO3 films in an electrolyte of H2SO4 at a 2 SCCM OFR is the highest DC of 1.007 × 10−7 cm2/s in comparison to crystalline WO3 films in an electrolyte of LiClO4 at a 6 SCCM OFR. The optical modulation of amorphous WO3 films in H2SO4 electrolyte solution is 86% at 6 SCCM OFR. In the CE results, crystalline WO3 films in an electrolyte solution of H2SO4 performed better in an electrochemical cell than amorphous WO3 films in an electrolyte solution of LiClO4. Finally, we reach the conclusion that the electrolyte solution of H2SO4 contributes to improving the electrochromic properties of WO3.
Data availability
All data generated or analyzed during this study are included in this article and are available from the author.
References
T. Brousse, D. Bélanger, K. Chiba, M. Egashira, F. Favier, J. Long, J.R. Miller, M. Morita, K. Naoi, P. Simon, and W. Sugimoto, Materials for electrochemical capacitors. Springer Handbooks (2017). https://doi.org/10.1007/978-3-662-46657-5_16.
R.S. Vemuri, M.H. Engelhard, and C.V. Ramana, Correlation between surface chemistry, density, and band gap in nanocrystalline WO3 thin films. ACS Appl. Mater. Interfaces 4, 1371 (2012). https://doi.org/10.1021/am2016409.
C.G. Granqvist, Oxide electrochromics: an introduction to devices and materials. Sol. Energy Mater. Sol. Cells 99, 1 (2012). https://doi.org/10.1016/j.solmat.2011.08.021.
J. Gupta, H. Shaik, and K.N. Kumar, A review on the prominence of porosity in tungsten oxide thin films for electrochromism. Ionics (Kiel) 27, 2307 (2021). https://doi.org/10.1007/s11581-021-04035-8.
J. Gutpa, H. Shaik, K.N. Kumar, and S. Abdul, Materials Science in Semiconductor Processing PVD techniques proffering avenues for fabrication of porous tungsten oxide (WO3) thin films : a review. Mater. Sci. Semicond. Process. 143, 106534 (2022). https://doi.org/10.1016/j.mssp.2022.106534.
M. Grätzel, Ultrafast colour displays. Nature 409, 575 (2001). https://doi.org/10.1038/35054655.
C.G. Granqvist, Electrochromics for smart windows: oxide-based thin films and devices. Thin Solid Films 564, 1 (2014). https://doi.org/10.1016/j.tsf.2014.02.002.
G.A. Niklasson and C.G. Granqvist, Electrochromics for smart windows :thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 17(2), 127 (2007). https://doi.org/10.1039/b612174h.
C.G. Granqvist, Electrochromic tungsten oxide films: review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 60, 201 (2000). https://doi.org/10.1016/S0927-0248(99)00088-4.
H. Simchi, B.E. McCandless, T. Meng, and W.N. Shafarman, Structural, optical, and surface properties of WO3 thin films for solar cells. J. Alloy. Compd. 617, 609 (2014). https://doi.org/10.1016/j.jallcom.2014.08.047.
Y.A.K. Reddy, B. Ajitha, A. Sreedhar, and E. Varrla, Enhanced UV photodetector performance in bi-layer TiO2/WO3 sputtered films. Appl. Surf. Sci. 494, 575 (2019). https://doi.org/10.1016/j.apsusc.2019.07.124.
A.V. Shchegolkov, S.H. Jang, A.V. Shchegolkov, Y.V. Rodionov, A.O. Sukhova, and M.S. Lipkin, A brief overview of electrochromic materials and related devices: a nanostructured materials perspective. Nanomaterials 11, 2376 (2021). https://doi.org/10.3390/nano11092376.
A.R. GV, H. Shaik, K.N. Kumar, V. Madhavi, H.D. Shetty, S.A. Sattar, M. Dhananjaya, B. Daruka Prasad, G.R. Kumar, and B.H. Doreswamy, Structural and electrochemical studies of WO3 coated TiO2 nanorod hybrid thin films for electrochromic applications. Optik 277, 170694 (2023). https://doi.org/10.1016/j.ijleo.2023.170694.
K. Naveen Kumar, H. Shaik, A. Pawar, L.N. Chandrashekar, S.A. Sattar, G. Nithya, R. Imran Jafri, V. Madhavi, J. Gupta, and G.V. Ashok Reddy, Effect of annealing and oxygen partial pressure on the RF sputtered WO3 thin films for electrochromic applications. Mater. Today: Proc. 59, 339 (2022). https://doi.org/10.1016/j.matpr.2021.11.185.
K. Naveen Kumar, H. Shaik, L.N. Chandrashekar, P. Aishwarya, S. Abdul Sattar, G. Nithya, V. Madhavi, R. Imran Jafri, J. Gupta, and G.V. Ashok Reddy, On ion transport during the electrochemical reaction on plane and GLAD deposited WO3 thin films. Mater. Today Proc. 59, 275 (2022). https://doi.org/10.1016/j.matpr.2021.11.113.
L. Geng, Gas sensitivity study of polypyrrole/WO3 hybrid materials to H2S. Synth. Met. 160, 1708 (2010). https://doi.org/10.1016/j.synthmet.2010.06.005.
M. Trapatseli, D. Vernardou, P. Tzanetakis, and E. Spanakis, Field emission properties of low-temperature, hydrothermally grown tungsten oxide. ACS Appl. Mater. Interfaces 3, 2726 (2011). https://doi.org/10.1021/am200519w.
R. Mukherjee and P.P. Sahay, Improved electrochromic performance in sprayed WO3 thin films upon Sb doping. J. Alloy. Compd. 660, 336 (2016). https://doi.org/10.1016/j.jallcom.2015.11.138.
N. Naseri, R. Azimirad, O. Akhavan, and A.Z. Moshfegh, Improved electrochromical properties of sol-gel WO3 thin films by doping gold nanocrystals. Thin Solid Films 518, 2250 (2010). https://doi.org/10.1016/j.tsf.2009.08.001.
J. Thangala, S. Vaddiraju, R. Bogale, R. Thurman, T. Powers, B. Deb, and M.K. Sunkara, Large-scale, hot-filament-assisted synthesis of tungsten oxide and related transition metal oxide nanowires. Small 3, 890 (2007). https://doi.org/10.1002/smll.200600689.
J. Zhang, X.L. Wang, X.H. Xia, C.D. Gu, Z.J. Zhao, and J.P. Tu, Enhanced electrochromic performance of macroporous WO3 films formed by anodic oxidation of DC-sputtered tungsten layers. Electrochim. Acta 55, 6953 (2010). https://doi.org/10.1016/j.electacta.2010.06.082.
K. Naveen Kumar, H. Shaik, Sathish, V. Madhavi, and S. Abdul Sattar, On the bonding and electrochemical performance of sputter deposited WO3 thin films. IOP Conf. Ser. Mater. Sci. Eng. 872, 012147 (2020). https://doi.org/10.1088/1757-899X/872/1/012147.
K.N. Kumar, S.A. Sattar, G.V. Ashok Reddy, R.I. Jafri, R. Premkumar, M.R. Meera, A.A. Ahamed, M. Muthukrishnan, M. Dhananjaya, and A.M. Tighezza, Structural, optical, and electrochromic properties of RT and annealed sputtered tungsten trioxide (WO3) thin films for electrochromic applications by using GLAD technique. J. Mater. Sci. Mater. Electron. 34, 1934 (2023). https://doi.org/10.1007/s10854-023-11285-x.
K.N. Kumar, G. Nithya, H. Shaik, L.N. Chandrashekar, P. Aishwarya, and A.S. Pawar, Optical and electrochromic properties of DC magnetron sputter deposited tungsten oxide thin films at different electrolyte concentrations and vertex potentials for smart window applications. J. Mater. Sci. Mater. Electron. 34, 789 (2023). https://doi.org/10.1007/s10854-023-10180-9.
A.R. GV, K.N. Kumar, S. Abdul, H.D. Shetty, N. Guru, R.I. Jafri, C. Devaraja, B.C. Manjunatha, C.S. Kaliprasad, R. Premkumar, and S. Ansar, Physica B: condensed Matter Effect of post annealing on DC magnetron sputtered tungsten oxide (WO3) thin films for smartwindow applications. Phys. B Condens Matter 664, 414996 (2023). https://doi.org/10.1016/j.physb.2023.414996.
K.N. Kumar, H. Shaik, J. Gupta, S. Abdul, I. Jafri, A. Pawar, V. Madhavi, A.R. GV, and G. Nithya, Sputter deposited tungsten oxide thin films and nanopillars: electrochromic perspective. Mater. Chem. Phys. 278, 125706 (2022). https://doi.org/10.1016/j.matchemphys.2022.125706.
K. Naveen Kumar, G. Nithya, H. Shaik, B. Hemanth, M. Chethana, K. Kishore, V. Madhavi, R.I. Jafri, S.A. Sattar, J. Gupta, and G.V. Ashok Reddy, Simulation and fabrication of tungsten oxide thin films for electrochromic applications. Phys. B Condens Matter 640, 413932 (2022). https://doi.org/10.1016/j.physb.2022.413932.
K.N. Kumar, S.A. Sattar, H. Shaik, A.R. GV, R.I. Jafri, M. Dhananjaya, A.S. Pawar, N.G. Prakash, R. Premkumar, S. Ansar, L.N. Chandrashekar, and P. Aishwarya, Effect of partial pressure of oxygen, target current, and annealing on DC sputtered tungsten oxide (WO3) thin films for electrochromic applications. Solid State Ion 399, 116275 (2023). https://doi.org/10.1016/j.ssi.2023.116275.
G.V. Ashok Reddy, H. Shaik, K. Naveen Kumar, R. Imran Jafri, S.A. Sattar, J. Gupta, and B.H. Doreswamy, Thickness dependent tungsten trioxide thin films deposited using DC magnetron sputtering for electrochromic applications. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2022.11.134.
K. Naveen Kumar, H. Shaik, V. Madhavi, R. Imran Jafri, J. Gupta, G. Nithya, S.A. Sattar, and G.V. Ashok Reddy, Glancing angle sputter deposited tungsten trioxide (WO3) thin films for electrochromic applications. Appl. Phys. A Mater. Sci. Process. 128, 1 (2022). https://doi.org/10.1007/s00339-022-06124-5.
G. Nithya, K.N. Kumar, R. Sai Yashwanth, W. Baig, S.V. Sai Ganesh, S. Hanvish Kumar, Simulation, deposition, and characterization of WO3 and multilayered WO3/Ag thin film structure for smart window applications, in: 2023 International Conference on Recent Advances in Science and Engineering Technology (ICRASET, IEEE, 2023), pp. 1–3. https://doi.org/10.1109/ICRASET59632.2023.10420072.
V. Madhavi, P. Kondaiah, H. Shaik, K.N. Kumar, T.S.S. Kumar Naik, G.M. Rao, and P.C. Ramamurthy, Fabrication of porous 1D WO3 NRs and WO3/BiVO4 hetero junction photoanode for efficient photoelectrochemical water splitting. Mater. Chem. Phys. 274, 125095 (2021). https://doi.org/10.1016/j.matchemphys.2021.125095.
K.N. Kumar, G. Nithya, H. Shaik, L.N. Chandrashekar, P. Aishwarya, and A.S. Pawar, Optical and electrochromic properties of DC magnetron sputter deposited tungsten oxide thin films at different electrolyte concentrations and vertex potentials for smart window applications. J. Mater. Sci. Mater. Electron. 34, 789 (2023). https://doi.org/10.1007/s10854-023-10180-9.
J. Gupta, H. Shaik, K.N. Kumar, S.A. Sattar, and G.V.A. Reddy, Optimization of deposition rate for E-beam fabricated tungsten oxide thin films towards profound electrochromic applications. Appl. Phys. A Mater. Sci. Process. 128, 1 (2022). https://doi.org/10.1007/s00339-022-05609-7.
J. Gutpa, H. Shaik, K. Naveen Kumar, and S.A. Sattar, Optimization of GLAD angle for E-beam-fabricated Tungsten oxide (WO3) thin films towards novel electrochromic behavior. J. Electron. Mater. (2022). https://doi.org/10.1007/s11664-022-10036-8.
J.Z. Ou, S. Balendhran, M.R. Field, D.G. McCulloch, A.S. Zoolfakar, R.A. Rani, S. Zhuiykov, A.P. O’Mullane, and K. Kalantar-Zadeh, The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties. Nanoscale 4, 5980 (2012). https://doi.org/10.1039/c2nr31203d.
J. Zhang, J.P. Tu, X.H. Xia, X.L. Wang, and C.D. Gu, Hydrothermally synthesized WO3 nanowire arrays with highly improved electrochromic performance. J. Mater. Chem. 21, 5492 (2011). https://doi.org/10.1039/c0jm04361c.
A.R. GV, K. Naveen Kumar, H. Shaik, H.D. Shetty, R. Imran Jafri, S. Abdul Sattar, K. Kamath, and B.H. Doreswamy, Growth of cerium oxide nanorods by hydrothermal method and electrochromic properties of CeO2/WO3 hybrid thin films for smart window applications. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2022.11.316.
G.V. Ashok Reddy, S.A. Sattar, K. Naveen Kumar, C.S. KaliPrasad, C. Devaraja, R. Imran Jafri, and B.H. Doreswamy, Effect of tungsten oxide thin films deposited on cerium oxide nano rods for electrochromic applications. Opt. Mater. 134, 113220 (2022). https://doi.org/10.1016/j.optmat.2022.113220.
A.R. GV, K.N. Kumar, H. Shaik, R.I. Jafri, R. Naik, and B.H. Doreswamy, Optical and electrochromic properties of CeO2/WO3 hybrid thin films prepared by hydrothermal and sputtering. Int. J. Eng. Trends Technol. 70, 1 (2022).
G.V. Ashok Reddy, K.N. Kumar, S.A. Sattar, N.G. Prakash, B. Daruka Prasad, M. Dhananjaya, G.R. Kumar, H.S. Yogananda, S.M. Hunagund, and S. Ansar, Structural, optical, and electrochromic properties of rare earth material (CeO2)/transitional metal oxide (WO3) thin film composite structure for electrochromic applications. Ionics (2023). https://doi.org/10.1007/s11581-023-05078-9.
G.F. Cai, J.P. Tu, D. Zhou, X.L. Wang, and C.D. Gu, Growth of vertically aligned hierarchical WO3 nano-architecture arrays on transparent conducting substrates with outstanding electrochromic performance. Sol. Energy Mater. Sol. Cells 124, 103 (2014). https://doi.org/10.1016/j.solmat.2014.01.042.
V. Madhavi, P. Kondaiah, O.M. Hussain, and S. Uthanna, Structural, optical, and luminescence properties of reactive magnetron sputtered tungsten oxide thin films. Int. Scholar. Res. Not. 2012, 1 (2012). https://doi.org/10.5402/2012/801468.
S.H. Mohamed, H.A. Mohamed, and H.A. Abd El Ghani, Development of structural and optical properties of WOx films upon increasing oxygen partial pressure during reactive sputtering. Phys. B 406, 831 (2011). https://doi.org/10.1016/j.physb.2010.12.005.
S.H. Lee, H.M. Cheong, C.E. Tracy, A. Mascarenhas, D.K. Benson, and S.K. Deb, Raman spectroscopic studies of electrochromic a-WO3. Electrochim. Acta 44, 3111 (1999). https://doi.org/10.1016/S0013-4686(99)00027-4.
J. Gabrusenoks, A. Veispals, A. Von Czarnowski, and K.H. Meiwes-Broer, Infrared and Raman spectroscopy of WO3 and CdWO4. Electrochim. Acta 46, 2229 (2001). https://doi.org/10.1016/S0013-4686(01)00364-4.
R. Chandra, A.K. Chawla, and P. Ayyub, Optical and structural properties of sputter-deposited nanocrystalline Cu2O films: effect of sputtering gas. J. Nanosci. Nanotechnol. 6, 1119 (2006). https://doi.org/10.1166/jnn.2006.176.
A.K. Chawla, S. Singhal, H.O. Gupta, and R. Chandra, Effect of sputtering gas on structural and optical properties of nanocrystalline tungsten oxide films. Thin Solid Films 517, 1042 (2008). https://doi.org/10.1016/j.tsf.2008.06.068.
M.B. Babu and K.V. Madhuri, Synthesis and electrochromic properties of nanocrystalline WO3 thin films. Phys. B Condens. Matter 584, 412068 (2020). https://doi.org/10.1016/j.physb.2020.412068.
C.K. Wang, D.R. Sahu, S.C. Wang, C.K. Lin, and J.L. Huang, Structural evolution and chemical bonds in electrochromic WO3 films during electrochemical cycles. J. Phys. D Appl. Phys. 45, 225303 (2012). https://doi.org/10.1088/0022-3727/45/22/225303.
K.J. Patel, G.G. Bhatt, S.S. Patel, R.R. Desai, J.R. Ray, C.J. Panchal, P. Suryavanshi, V.A. Kheraj, and A.S. Opanasyuk, Thickness-dependent electrochromic properties of amorphous tungsten trioxide thin films. J. Nano Electron. Phys. 9, 03040 (2017). https://doi.org/10.21272/jnep.9(3).03040.
V. Madhavi, P. Kondaiah, O.M. Hussain, and S. Uthanna, Structural, optical and electrochromic properties of RF magnetron sputtered WO3 thin films. Phys. B 454, 141 (2014). https://doi.org/10.1016/j.physb.2014.07.029.
C.S. Hsu, C.C. Chan, H.T. Huang, C.H. Peng, and W.C. Hsu, Electrochromic properties of nanocrystalline MoO3 thin films. Thin Solid Films 516, 4839 (2008). https://doi.org/10.1016/j.tsf.2007.09.019.
B. Wen-Cheun Au, A. Tamang, D. Knipp, and K.Y. Chan, Post-annealing effect on the electrochromic properties of WO3 films. Opt. Mater. 108, 110426 (2020). https://doi.org/10.1016/j.optmat.2020.110426.
X. Sun, Z. Liu, and H. Cao, Electrochromic properties of N-doped tungsten oxide thin films prepared by reactive DC-pulsed sputtering. Thin Solid Films 519, 3032 (2011). https://doi.org/10.1016/j.tsf.2010.12.017.
Acknowledgments
The authors thank the Nitte Meenakshi Institute of Technology, Bengaluru, India, for providing facilities, and the work was supported by the Researchers Supporting Project number (RSPD2023R765), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, K.N., Reddy, G.V.A., Sattar, S.A. et al. Effect of Oxygen Flow Rate, Post-annealing Temperature, and Different Electrolyte Concentrations on WO3 Thin Films Deposited by DC Magnetron Sputtering For Electrochromic Applications. J. Electron. Mater. 53, 2351–2366 (2024). https://doi.org/10.1007/s11664-024-11000-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-11000-4