Abstract
The atomic structures, growth patterns, new spintronic, magneto-optic, and electrical properties of 2D armchair nanoribbons of group-IV transition metal nitrides (TiN, ZrN, and HfN) have been investigated. The atomic unit cells of TiN, ZrN, HfN, TiZrN, TiHfN, and ZrHfN have been transformed into the matching armchair nanoribbons. By comparing the proposed nanoribbon to the bulk and monolayer structures of these metal nitrides, the structural stability, density, and projected density of states, band structure, magnetic behavior, and polarization were evaluated. The outcomes demonstrate that, in comparison to the crystalline structure, the suggested systems exhibit ferrimagnetic behavior and a remarkable magnetic moment. Also, a higher magnetic moment of about 24 μB in the supercells of the suggested nanoribbons was discovered due to the increased atom count. The proposed structures demonstrated indirect band gap semiconductor capabilities in the range between 1.66 eV and 2.16 eV which is again an unknown phenomenon in the bulk structure of transition metal nitrides. Owing to these characteristics, the proposed structures can be employed in many optoelectronic applications, such as solar cell devices, LEDs, laser diodes, and optical fibers where the band gap is the key requirement. Apart from this, they can also be useful in coating biomedical implanted chips, transformers, switches, and batteries. Memory storage devices will also benefit from the unique characteristics of the proposed nanoribbon structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few years, there has been a growing fascination with advanced stubborn metallic nitrides that have various uses, including appealing capability gadgets and hindrances to diffusion.1,2 This applies especially to titanium nitride (TiN), which is used extensively as protection for cutting and framing gadgets because of its noted characteristics of high hardness, resistance to corrosion, excellent heat stability, and oxidation resistance.3 A TiN coating can be applied to a variety of tools, including taps, drills, reamers, end mills, dovetails, and punching and shaping tools. Metallic nitrides that show progress possess characteristics that combine those of both steel and non-steel materials due to their shared covalent, ionic, and metallic bonding properties.1,2 TiN films are utilized in scientific instruments as well as metals for hip joint replacements, dental prostheses, and heart valve replacements, due to their excellent hemo-compatibility and innate bio-compatibility. To improve the wear and corrosion resistance of titanium implants, there is a process of producing TiN layers on their surface. Due to their remarkable biostability, TiN coatings are suitable for application as electrodes in bioelectronic devices, advanced prosthetics, and biosensors that need to withstand corrosion from body fluids.
The substances used in various biomedical applications, like retina implants and neuroprosthetic implants,4,5 as well as biomedical micro-electro-mechanical structures, involve the insertion of an electrode chip into the body for endoscopic treatments that prioritize exceptional structural and thermal stability. Even though the conventionally used TiN is good for coating, due to the overlapped band gap properties it is not desirable for controlled electronic and optical characteristics required for implanting applications. In later research, the direct band gap semiconducting transition metal dichalcogenides and molybdenum disulfide (MoS2) have been commonly utilized in unique optoelectronic packages consisting of photo-transistors or photo-diodes according to sources.6,7,8,9,10 Nevertheless, photo-transistors based on MoS2 have shown very poor absorption and sensitivity levels which were moderate in a negative sense.11,12
Our previous research analyzing bulk structures of TiN, hafnium nitride (HfN), and zirconium nitride (ZrN) showed that HfN is the most favorable and promising candidate due to its remarkable thermal and mechanical properties.13,14 Even in the monolayer structure of these metal nitrides, HfN demonstrated better thermal and mechanical stability than TiN. Nevertheless, the issue these metallic nitrides face, including HfN, is the presence of an overlapped band gap. Furthermore, the bulk structures of these metal nitrides do not exhibit any magnetic moment, which limits their utilization in distinctive optoelectronic applications. The atomic chain structures of these metal nitrides, however, has shown a small magnetic moment, but, with the negligible indirect band gap, their applications have been very limited.15 This work has been carried out to explore new material structures that can not only provide the solution for controlled electronic behavior but also be stable against any temperature and environmental changes.
Hence, in this investigation, the bulk structures of TiN, ZrN, HfN, TiZrN, TiHfN, and ZrHfN have been converted to nanoribbons with an armchair structure. To analyze their electronic characteristics, the density of states (DOS), projected density of states (PDOS), and charge densities have been considered. Further, for thermal and dynamic stability, the cohesive energy, formation enthalpy, and phonon plots have been calculated. The polarization behavior has also been analyzed based on the individual contribution of atoms.
Methodology
The "Spanish initiative for Electronic simulations with thousands of atoms" (SIESTA)16 implementation of density-functional theory (DFT) was used in this work.17 On the aspect of the localized atomic orbitals, double Zeta plus single polarization orbitals have been utilized.18 For accurate measurement, the Perdew–Burke–Ernzerh scheme has been implemented using the generalized gradient approximation.
To model, a nanoribbon of a 1 × 4 × 3 supercell made up of 48 atoms called as armchair nanoribbon (anr) was utilized and hence the convention of proposed supercell structures is TiN_anr, ZrN_anr, HfN_anr, TiZrN_anr, TiHfN_anr, and ZrHfN_anr throughout this paper. Mesh cutoff, k-elements (inside the Monkhorst pack scheme), and lattice vector optimization for the structures under examination were three consecutive steps of the optimization. The computations used an acquired energy shift of 0.03 Ry and an augmented charge density energy mesh cutoff of 150 Ry, spin polarization true and non-collinear, electronic temperature 50 meV, SCF iteration, and CG step 300, and the force was 0.005 eV/Å setting for all the supercells in an armchair position. For sampling, a Monkhorst pack 1 × 12 × 12 has been employed. The geometry relaxations were completed when each atomic detail's forces appeared to be less than 0.001 eV/Å. Norm-preserving pseudopotentials were used as a resource to implicitly handle the center electrons,19 together with relativistic adjustments.
Cohesive energy ("Ec") and enthalpy ("∆H") calculations were utilized to assess the stability of the system after the optimization procedure. Many characteristics about the structure, chemical reactivity, and stability of solids are revealed by the magnitude of cohesive energy.20 The formation enthalpy primarily reveals the system's overall thermal stability. The following equations were used for calculations of "Ec" and "∆H":
where M stands for the metal, i.e., Ti, Zr, or Hf, and N stands for the nitrogen atom.
For the structures under study, phonon computations have also made sure that the structure is dynamically stable. The conjugate gradient approach to optimum systems dynamic balance should be based on the evaluation of phonon modes. The optical and acoustic phonon frequencies were estimated using a VIBRA tool and the pressure regular method,21 while SIESTA was used to calculate the forces. A total of 200 atoms were considered in the calculation of the phonon modes. By combining the segment elements with the pressure constants within this larger system, phonon dispersions were created.
Result and Discussion
Structural Properties and Stability
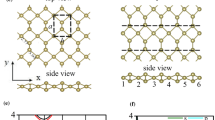
Figure 1a–f indicates the nanoribbon unit cell of the bulk structure. In (a), (b), (c) a single atom of Ti, Hf, and Zr, respectively, is bonded with two N atoms, and in (d), (e), and (f), a combination pair of these atoms (Ti, Hf, and Zr, respectively) is bonded with single N atoms. These unit cell structures were converted into the armchair nanoribbons of TiN_anr, ZrN_anr, HfN_anr, TiZrN_anr, TiHfN_anr, and ZrHfN_anr, as depicted in Fig. 2a–f.
Table I shows the important structural and stability parameters of the proposed unit cell and supercell nanoribbon structures. The values of bond lengths and bond angles show that both the unit cell and supercell structures of the proposed nanoribbon structures are stable and do not distort the system. The cohesive energy, Ec, and the enthalpy of formation, ΔH, for the nanoribbon system under consideration (calculated using Eqs. 1 and 2) are also shown in Table I. It can be seen that the unit cell and supercell of the HfN nanoribbon is the most negative among the structures. In other words, the bonding between the atoms of the HfN nanoribbon is the highest and hence the stability against any environmental and temperature changes is also superior compared with the other structures under study.
The phonon scattering curves for the suggested nanoribbon structures are shown in Fig. 3. The BZ and phonon dispersion curves' positive values point to strong, advantageous structural characteristics. While the phonon dispersion curves for the LA and TA branches are known to be linear, as K approaches to 0, the process of the ZA branch is quadratic due to the quick reduction of the transverse force close to the point. The highest optical domain degrades in the gamma region, as shown in Fig. 3. Yet, in HfN, ZrN, and ZrHfN, LO-TO separation was more obvious. The observed advantages of the cohesive energy, formation enthalpy, and phonon scattering curves suggest that the proposed structures are stable against any unfavorable changes in the thermal, dynamic, and environmental conditions.
Electronic Properties
A material's electronic behavior can be accurately predicted using DOS. The DOS pattern can be used to quickly comprehend a substance's physical and chemical properties. Figure 4(a) and (b) demonstrates the DOS pattern for the structures under investigation. The Fermi level was moved to zero to plot the data. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) points are distinct, as seen in Fig. 4, with the majority of the peaks shifting to the conduction band. As a result, the proposed nanoribbon structures behave as n-type indirect semiconductors.
Energy band diagrams were also examined in order to better delve into the electronic behavior of the proposed unit cell and supercell nanoribbon structures. The band diagram in Fig. 5 displays good agreement with the provided DOS characteristics. It is clear that the unit cell and supercell display band gap data and depict the behavior of an n-type indirect semiconductor. Table I also includes the band gap magnitudes for the proposed structures.
Magnetic Properties
The magnetic moment is the second significant shift in the proposed nanoribbon structures' attributes that has been noticed. TiN, ZrN, and HfN all have non-magnetic bulk structures. Nonetheless, it is clear from Fig. 6 that the proposed structures exhibit magnetism because of the variation in the up-spin and down-spin of the DOS. The PDOS profiles were also examined, as shown in Figs. 7 and 8, to better understand the individual contributions of atomic orbitals to the magnetic moment of the proposed nanoribbons.
It can be seen from the PDOS profiles of TiN, ZrN, HfN, TiZrN, TiHfN, and ZrHfN nanoribbon unit cells (Fig. 7) and the TiN anr, ZrN anr, HfN anr, TiZrN anr, TiHfN anr, and ZrHfN anr nanoribbon supercells (Fig. 8) that the difference in the spin-up and spin-down states are higher in the N − 2 s and M (Ti, Zr, and Hf) − 4d elements. The magnetic moment revealed in this work is also a result of the electron's spin and orbital angular locations.
As demonstrated in Figs. 7 and 8, the armchair nanoribbon structures' valence band has a larger electron density in the down region, particularly between − 20 and 0 eV. Because of the disparity in densities between the top and bottom of the conduction band, the electron spin is lower. There is a magnetic moment introduced by this up-and-down state difference. These transition metal nitrides, in particular, lack any magnetic characteristics in their bulk forms. It is interesting to note that the proposed nanoribbon structures exhibit a strong magnetic moment without the need of any dopant or external material. The magnetic moment of the proposed nanoribbon unit cell, which is roughly 2 μB, rises to 23–24 μB in the supercell nanoribbon structures, as shown in Table I. As illustrated in Fig. 1, each unit cell has a total of four atoms, and each nanoribbon (Fig. 2) contains 12 times as many atoms as a unit cell, resulting in an increase in the magnetic moment (ratio of 1:12). This improves the reported magnetic moments' consistency even more.
The type of atom–atom bonding was thoroughly examined in all of the supercell formations. Plots of charge density have been plotted for that purpose, as seen in Fig. 9. The graphic makes it obvious that the bonds are ionic in nature. These materials are also magnetic because of the ionic structure of the chain. In particular, the PDOS profiles of the suggested systems' magnetic moments have previously been established. Also, this displays the band structure of this study as well as the features of the semiconductor that have already been revealed in the DOS. In order to determine the sort of magnetism created in the material under research, the polarization direction of the magnetic moment was also investigated. The suggested nanoribbon structures exhibit ferrimagnetic behavior with the opposite direction of polarization between the metal and nitrogen atoms, as seen in Figs. 10 and 11.
Discussion on Possible Applications
The comparison between the proposed nanoribbons and the bulk forms of TiN, ZrN, and HfN is summarized in Table II. The high values of cohesive energy and formation enthalpy show that the suggested nanoribbon structures are not only stable but also more resistant to environmental changes and temperature fluctuations than bulk structures. Since implanted devices are to be placed in an isolated environment inside the body, they are good alternatives for endoluminal and neuroprosthetic implants. Due to the risk of catastrophic health problems brought on by the heat generated in these circumstances, temperature stability is a major concern. The proposed nanoribbon structures can take the place of the current bulky framework used to make such implanted devices, and they can even serve as a shield for an electronic chip that is inserted for patient safety. Furthermore, memory storage applications can make use of their distinctive ferrimagnetic behavior. They can be employed in microwave frequency systems and computer memory cores due to their reduced eddy current losses. They can also be utilized in electrical circuits as insulators.28
Specifically, with the most stable values of formation enthalpy and cohesive energy, the HfN nanoribbon can be the best choice for biomedical applications among all the nanoribbons. The TiN nanoribbon consists of the highest band gap and is hence found most suitable for any optoelectronic applications where the band gap is the primary screening parameter. However, since all the nanoribbons showed a similar magnetic moment for memory storage applications, any of them can be deployed.
Conclusions
Armchair nanoribbon structures have been proposed and investigated by converting the atomic chain of TiN to TiN anr, ZrN anr, HfN anr, TiZrN anr, TiHfN anr, and ZrHfN anr, based on the first-principles values of density functional theory. The proposed chain structure offers great abrasion and temperature stability which is desired in the construction of electrodes useful in various biomedical applications, such as retinal and neuroprosthetic implants. The investigation also revealed the behavior of the proposed nanoribbon structures as n-type indirect semiconductors, useful for solar cell devices and electronic industries. The materials also exhibit magnetic properties in terms of values and amplitudes, according to the DOS–PDOS profiles, which are useful for memory storage devices. Overall, the proposed nanoribbon structures are advantageous for biological, optoelectronic, magnetic, magneto-optical, and spintronic applications due to their unique properties. We anticipate that our work will offer fresh perspectives to scientists studying 2D materials, and serve as a conceptual framework for enhancing material performance.
References
H. J. Goldschmidt, in Interstitial Alloys, edited by H. J. Goldschmidt (Springer US, Boston, MA, 1967), pp. 14–59.
K.K. Korir, G.O. Amolo, N.W. Makau, and D.P. Joubert, Diamond Rel. Miner. 20, 157 (2011).
M.G. Faga, G. Gautier, R. Calzavarini, M. Perucca, E.A. Boot, F. Cartasegna, and L. Settineri, Wear 263, 1306 (2007).
A. Tiwari, and R.H. Talwekar, IETE J. Res. 65, 172 (2019).
A. Tiwari, and R.H. Talwekar, J. Adv. Res. Dyn. Control Syst. 9, 1 (2017).
H. Wang, C. Li, P. Fang, Z. Zhang, and J.Z. Zhang, Chem. Soc. Rev. 47, 6101 (2018).
J.A. Wilson and A.D. Yoffe, Adv. Phys. 18, 193 (1969).
A.H. Castro Neto, Phys. Rev. Lett. 86, 4382 (2001).
R. H. Talwekar and A. Tiwari, in 2021 IEEE 6th International Conference on Computing, Communication and Automation (ICCCA) (2021), pp. 672–677.
R.H. Talwekar and A. Tiwari, Mater. Today Proc. 59, 692 (2022).
B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti, and A. Kis, Nat. Nanotechnol. 6, 147 (2011).
J. Miao, W. Hu, Y. Jing, W. Luo, L. Liao, A. Pan, S. Wu, J. Cheng, X. Chen, and W. Lu, Small 11, 2392 (2015).
A. Tiwari, R.H. Talwekar, and M.L. Verma, J. Electron. Mater. 1, 1 (2021).
M.L. Verma and A. Tiwari, Chem. Phys. Lett. 781, 138992 (2021).
N.K. Verma, S.K. Srivastava, M.L. Verma, and A. Tiwari, Mater. Chem. Phys. 293, 126945 (2023).
J.M. Soler, E. Artacho, J.D. Gale, A. Garcia, J. Junquera, P. Ordejon, and D.S. Portal, J. Phys. Condens. Matter 14, 2745 (2002).
E. Rudberg, E.H. Rubensson, and P. Sałek, J. Chem. Theory Comput. 7, 340 (2011).
R.Q. Zhang, Q.Z. Zhang, and M.W. Zhao, Theor. Chem. Acc. 112, 158 (2004).
N. Troullier and J.L. Martins, Phys. Rev. B 43, 1993 (1991).
A.S. Verma, B.K. Sarkar, and V.K. Jindal, Cohesive Energy of Zincblende (A III B V and A II B VI ) Structured Solids (2010).
A. Bilić and J.D. Gale, Phys. Rev. B Condens Matter Mater. Phys. 79, 1 (2009).
K. Chen, L.R. Zhao, J. Rodgers, and J.S. Tse, J. Phys. D Appl. Phys. 36, 2725 (2003).
M. Zhang, K. Cheng, H. Yan, Q. Wei, and B. Zheng, Sci. Rep. 6, 36911 (2016).
M. Magnuson, M. Mattesini, S. Li, C. Höglund, M. Beckers, L. Hultman, and O. Eriksson, Phys. Rev. B 76, 195127 (2007).
Z. Erjun, W. Jinping, M. Jian, and W. Zhijian, Comput. Mater. Sci. 47, 1064 (2010).
A. Srivastava, M. Chauhan, and R.K. Singh, Physica Status Solidi B - Basic Solid State Phys. 248, 2793 (2011).
S. Yu, Q. Zeng, A.R. Oganov, G. Frapper, B. Huang, H. Niu, and L. Zhang, RSC Adv. 7, 4697 (2017).
A. Tiwari and A. K. Sahu, in 2012 National Conference on Computing and Communication Systems (2012), pp. 1–6.
Acknowledgments
This project is supported by "Shri Shankaracharya Technical Campus", Bhilai under the great supervision of "Dr. Mohan Lal Verma" and "Dr. Ashish Tiwari" (G H Raisoni College of Engineering and Management, Pune). The author acknowledges the support of “Vice Chancellor” of Bundelkhand University, Jhansi, for providing him the opportunity to complete his PhD work.
Funding
This project is not funded by any of the agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, N.K., Bhadoria, B.S., Verma, M.L. et al. A DFT Study on Armchair Nanoribbon Structures of TiN, ZrN, and HfN. J. Electron. Mater. 53, 2367–2381 (2024). https://doi.org/10.1007/s11664-024-10978-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10978-1