Abstract
The rapidly developing field of wireless communication technology requires advanced microwave dielectric ceramic materials. Here, spinel-structured MgGa2O4 and ZnGa2O4 ceramics were prepared using a reaction sintering method. The effect of sintering temperature was investigated with respect to the crystal structure, microstructure, and microwave dielectric properties of MgGa2O4 and ZnGa2O4 ceramics. Rietveld refinement analysis and quality factor (Q×f) values suggested that the occupancy sites and percentage of cations in MgGa2O4 would affect the microwave dielectric properties. Microstructure analysis indicated that abnormal grain growth at higher sintering temperatures would degrade the microwave dielectric properties of ZnGa2O4 ceramics. MgGa2O4 ceramics sintered at 1610°C exhibited promising microwave dielectric properties, i.e., εr = 9.4, Q×f = 167,500 GHz, and τf = −63 ppm/°C, while ZnGa2O4 ceramics sintered at 1550°C possessed a higher εr and a lower Q×f value of 10.5 and 126,900 GHz, respectively, and τf = −60 ppm/°C. The τf values of MgGa2O4 and ZnGa2O4 ceramics were tuned to near-zero levels by adding 8 mol.% CaTiO3. These results indicate that MgGa2O4 and ZnGa2O4 ceramics produced by reaction sintering show promise for applications in the field of 5G communication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural spinel is a mineral mainly consisting of magnesium, aluminum, and oxygen elements, and it belongs to a cubic crystal system with space group Fd-3 m (no. 227). This crystal structure is called a spinel structure. Transition metal elements such as chromium, manganese, iron, and zinc also often exist in natural spinel, and endow the ore with brilliant colors and special physical and chemical properties.1,2,3 The common chemical formula is AB2O4, where A and B are divalent and trivalent cations, respectively. There are three different structures in spinel depending on the occupancy sites of cations. A normal spinel structure is one in which A and B ions occupy the tetrahedral (8a) and octahedral (16d) sites, respectively. It is called an inverse spinel structure when A and half of B ions occupy the 16d sites, and the remaining half of B ions occupy the 8a sites. Most spinel-structured compounds have a mixed structure. In the mixed structure, A and B ions are randomly distributed at 8a and 16d sites. This structure is usually expressed as (A1−xBx) [AxB2−x]O4, where x is the degree of inversion.4,5
Microwave dielectric ceramics (MWDCs) are one of the key classes of materials for modern communication applications. MWDCs are widely used to prepare dielectric resonators, filters, and antennas.6,7 To meet the requirements for high-frequency data transmission, namely lower delay time, smaller signal loss, and good temperature stability, MWDCs with a low dielectric constant (εr), high quality factor (Q×f), and near-zero temperature coefficient of the resonant frequency (τf) are desired. It has been shown that some spinel-structured compounds have excellent microwave dielectric properties. Shannon and Rossman reported dielectric constants and dielectric loss (tanδ) for MgAl2O4 single crystals of εr = 8.325 and tanδ = 0.0008 (@1 MHz).8 Sebastian et al. reported the microwave dielectric properties of MgAl2O4 (εr = 8.75, Q×f = 68,900 GHz, τf = −75 ppm/°C) and ZnAl2O4 (εr = 8.5, Q×f = 56,300 GHz, τf = −79 ppm/°C) ceramics produced by the conventional solid-state sintering method, and τf was adjusted to a near-zero value by the addition of TiO2.9,10 Many studies have since been conducted in which the microwave dielectric properties of MgAl2O4 and ZnAl2O4 ceramics were regulated by A-site and B-site ion substitution,11,12,13 and by forming composites.14,15 In contrast, reports on the microwave dielectric properties of spinel MgGa2O4 and ZnGa2O4 ceramics are relatively sparse. Previous studies have indicated that MgAl2O4, ZnAl2O4, and ZnGa2O4 are normal spinel-structured compounds, while MgGa2O4 exhibits a mixed structure.16 In 2013, the attractive microwave dielectric properties of MgGa2O4 (εr = 9.2, Q×f = 298,000 GHz, τf = −63.5 ppm/°C) and ZnGa2O4 (εr = 10.4, Q×f = 94,600 GHz, τf = −27 ppm/°C) ceramics were reported by Kan et al.5 and Xue et al.,17 respectively. In the study by Kan and coworkers, the relationship between the cation distributions and the microwave dielectric properties was considered, and the results indicated that the preferential site occupancy of Mg2+ and Ga3+ would affect the Q×f values. Cation substitution and composite strategies have subsequently been used to improve the performance of MgGa2O4 and ZnGa2O4 ceramics.18,19,20,21 However, these ceramics were prepared by the conventional solid-state sintering method. As is well known, the traditional solid-state sintering method involves complicated steps and causes the loss of raw materials, both of which increase the cost. In comparison, the reaction sintering method is a one-step sintering process that saves time and cost.22,23,24
In this work, MgGa2O4 and ZnGa2O4 ceramics were prepared using the reaction sintering method. The crystal structure, microstructure, and microwave dielectric properties of MgGa2O4 and ZnGa2O4 ceramics were studied and compared.
Experimental
Samples of MgGa2O4 (MGO) and ZnGa2O4 (ZGO) ceramics were prepared by the reaction sintering method. Ga2O3 (99.99%, Aladdin), MgO (99.99%, Aladdin), and ZnO (99.99%, Aladdin) powders were weighed in a molar ratio of 1:1 for MgGa2O4 and ZnGa2O4, respectively. Before weighing, the MgO powder was calcined at 900°C for 2 h to remove moisture. Two batches of powder were mixed by ball milling for 6 h, using zirconia balls and ethanol as milling and dispersion media, respectively. The slurry was then dried at 80°C for 12 h in an air oven. The dried powder was sieved through a 100-mesh screen. Later, the powder was firstly pressed into cylinders 12 mm in diameter and about 6 mm in height with a stainless steel mold. These cylinders were then further compacted via cold isostatic pressing at 200 MPa. Subsequently, the samples were sintered at 1400–1640°C for 4 h with a heating rate of 5°C/min in air.
The crystal structure of the sintered ceramics was identified by x-ray diffraction (XRD, X′Pert PRO, PANalytical, Netherlands) with Cu Kα radiation (λ = 0.15406 nm). Rietveld refinement analysis was applied to evaluate the cation distribution of the partially inverse spinel. The surface morphology of the samples was examined using scanning electron microscopy (SEM, FEI Inspect F, UK). Prior to surface examination, specimens were polished in a polishing machine (UNIPOL-1200S, KJ Group, China). Abrasive papers with grit sizes of 400, 800, 1200, and 2000 mesh were sequentially employed. The polished ceramics were thermally etched for 20 min at a temperature 50°C lower than the optimal sintering temperature. Raman spectra of MgGa2O4 and ZnGa2O4 ceramics were collected by a laser confocal spectrometer (LabRAM Odyssey, HORIBA, France) with a 532 nm laser. The microstructure of samples was also studied via transmission electron microscopy (TEM, FEI Tecnai G2 F20 S-TWIN, USA). The apparent density was measured by Archimedes' method. The theoretical density was referenced to the Rietveld refinement result. The relative density was the ratio of the apparent density to the theoretical density. The εr and Q×f values were measured by the Hakki–Coleman method25 using a vector network analyzer (E5071C, Agilent). The τf was obtained in a temperature chamber based on the following equation:
where f25 and f85 are the resonant frequencies at 25°C and 85°C, respectively, and ΔT is the temperature difference.
Results and Discussion
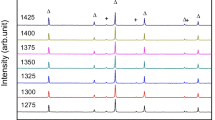
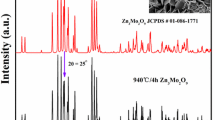
Figure 1a and b show the XRD patterns of the MgO-Ga2O3 and ZnO-Ga2O3 mixtures, respectively. In both mixtures, the rhombohedral Ga2O3 phase is detected alongside MgO, ZnO, and monoclinic Ga2O3 phases from the raw powders. This new Ga2O3 phase is from the phase transformation of monoclinic Ga2O3 during ball milling, consistent with the findings reported by Luchechko et al.26 For the MGO ceramics sintered at 1400~1640°C (see Fig. 1c), all diffraction peaks match well with the standard card of MgGa2O4 (JCPDS #10-0113). Similarly, the primary phase in ZGO ceramics corresponds to the JCPDS card (no. 38-1240) of ZnGa2O4, as shown in Fig. 1d. Notably, unlike MGO ceramics, ZGO ceramics sintered above 1550°C reveal the presence of the monoclinic Ga2O3 phase (JCPDS #43-1012), marked with an asterisk in Fig. 1d. This phenomenon can be attributed to the volatilization of zinc elements.
The exemplary refined XRD profiles of MGO and ZGO ceramics are displayed in Fig. 2. The structural parameters, cation distribution, and reliability factors are listed in Table I. In the case of MGO ceramics, the lattice parameter (a) and cell volume (V) exhibit a gradual increase. This trend appears to be linked to variations in the degree of inversion, x. The degree of inversion of MGO ceramics decreases as the sintering temperature increases, and remains nearly constant above 1600°C. This variation is in line with a previous report.5 Based on the goodness-of-fit indexes at the bottom of Table I, the refinement results are reliable. To clarify the significant changes in the lattice parameters, the bond strength (s) and covalence (fc) of MO4 tetrahedron and MO6 octahedron (M = Mg, Ga) were calculated using Eqs. 2 and 3:27,28
where s is the cation–oxygen bond strength, and R is the bond length in the MO4 tetrahedron and MO6 octahedron (see Table I). R1 (1.622 for Mg2+, 1.746 for Ga3+) and N (4.29 for Mg2+, 6.05 for Ga3+) are empirical parameters. a and M represent empirical constants which depend on the number of electrons. For Mg2+, a and M are 0.54 and 1.64, while for Ga3+ they are 0.60 and 1.50, respectively. The covalence of the cation–oxygen bond determined from the fc/s ratio in MO4 tetrahedron and MO6 octahedron is depicted in Fig. 3. The covalence of the cation–oxygen bonds in MO4 tetrahedron increases with increasing sintering temperature, while that in MO6 octahedron decreases, corresponding to a reduction in tetrahedral volume and an increase in octahedral volume, respectively. The increase in octahedral volume appears to dominate the change in cell parameters. The preferential occupation of cations would alter the polyhedral volume,29 driven by variations in the degree of inversion. In this study, Mg2+ tends to occupy the A site, while Ga3+ occupies the B site, with the increase in sintering temperature, ultimately influencing the cell parameters.
The SEM micrographs and the variation in average grain size for MGO and ZGO ceramic samples are shown in Fig. 4. Pores are evident for MGO and ZGO ceramics sintered at lower temperatures. For MGO ceramics, the microstructure becomes dense with increasing temperature, and no pores are observed in the sample sintered at 1610°C. In comparison, the number of pores reduces with elevated sintering temperatures in ZGO ceramics, but complete elimination is not achieved. Pores in Fig. 4e are distributed along grain boundaries. At 1490°C (Fig. 4f), a large number of pores are located inside the grain in the ZGO ceramic, attributed to the rapid movement of grain boundaries during grain growth, preventing the stomata from being discharged completely. At higher sintering temperatures (Fig. 4g and h), there are obviously fewer pores, which may originate from the grain annexation causing the grain boundaries to shift again. The heterogeneity of grains is also apparent in Fig. 4g. In other words, the anomalous grain growth appears in ZGO ceramics sintered at 1490°C and higher temperatures. Additionally, a step-like structure appears on the surfaces of ZGO ceramics. This can be ascribed to the volatilization of zinc.17 As shown in Fig. 4i, the ZGO ceramics have larger average grain sizes than MGO ceramics for each holding temperature. The average grain sizes of MGO and ZGO ceramics sintered at the highest temperature are 30.3 μm and 35.5 μm, respectively.
Group theory analysis has revealed that there are 42 vibrational modes at k = 0 in a primitive cell of the spinel. Among these modes, the Raman-active modes are A1g + 3T2g + Eg.30,31 The Raman spectra of MGO and ZGO ceramics are shown in Fig. 5a and b, respectively, which include the spectra of surfaces and cross sections. For the MGO ceramic sintered at 1610°C, all five Raman modes are observed on both the surface and cross section. However, the spectra of the surface and fracture surface in the ZGO ceramic sintered at 1550°C exhibit noticeable differences. Three of five Raman-active modes are detected on the surface and cross section. Apart from the three peaks in common, extra peaks are observed on the surface of the ZGO ceramic which are assigned to the vibrational modes of β-Ga2O3.32 These peaks mainly involve three regions: (i) liberation and translation of tetrahedron–octahedron chains (below 300 cm−1), (ii) deformation of Ga-O octahedron (~480–300 cm−1), and (iii) stretching and bending of Ga-O tetrahedron (~770–500 cm−1).33 No other peaks are noted on the cross section. This further indicates that the process of Zn volatilization mainly occurs on the surfaces, as shown in Fig. 4e–h.
To evaluate the crystallinity of MGO and ZGO ceramics under reaction sintering conditions, TEM tests were conducted. The low- and high-magnification images and corresponding selected-area electron diffraction (SAED) patterns are shown in Fig. 6. The high-resolution lattice images of MGO and ZGO ceramics (Fig. 6b and d) reveal excellent crystallinity characterized by a tight and regular arrangement of atoms. The SAED patterns, displaying clear dot matrices, further confirm the high crystallinity. The interplanar spacings of the \(\left(1\overline{1 }\overline{1 }\right)\) and \(\left(\overline{1 }1\overline{1 }\right)\) lattice planes for MGO ceramic are 0.484 nm and 0.485 nm, respectively. With regard to the faces with the same Miller indices for ZGO ceramic, the interplanar spacings are 0.487 nm and 0.485 nm, respectively.
The evolution of densities and microwave dielectric properties of MGO and ZGO ceramics versus sintering temperature is shown in Fig. 7. In Fig. 7a, ZGO ceramics have larger relative densities than MGO ceramics below 1520°C, which can be attributed to the more sufficient driving force from the exothermic reaction between ZnO and Ga2O3.34 MGO and ZGO ceramics achieve the largest relative densities of 96.1% and 95.6%, respectively, at 1580°C and 1550°C. At higher temperatures, the relative densities of MGO ceramics remain nearly constant, while those of ZGO ceramics decline to a certain extent, which is most likely due to the violent volatilization of Zn. For both ceramics, εr and Q×f values initially increase and then decrease, as shown in Fig. 7b and c. The highest εr values are 9.4 for the MGO ceramic and 10.5 for the ZGO ceramic, while the highest Q×f values are 167,500 GHz and 126,900 GHz, respectively. Many studies have pointed out that the Q×f value is closely related to relative density.35,36 A higher Q×f value is often accompanied by higher density. In the case of MGO ceramics, even when the density no longer increases, Q×f values continue to increase with sintering temperature, possibly due to a decrease in the degree of inversion. As noted by Kan et al., the reduction of the degree of inversion may improve the sinterability of MGO ceramics and enhance the crystallinity, resulting in a high Q×f value.5 Conversely, the deterioration of Q×f values in ZGO ceramics is mainly attributed to the structural defects, including Zn volatilization and abnormal grain growth. Generally, a dense and uniform microstructure can reduce the loss, thereby improving Q×f values.37 Their |τf| values first decrease with the increase in sintering temperature. Finally, the τf values for MGO ceramics fluctuate around −65 ppm/°C, while those for ZGO ceramics stabilize at −60 ppm/°C, as depicted in Fig. 7d.
In addition, the τf values of MGO and ZGO ceramics were tuned by adding CaTiO3 with a positive τf value (~ +800 ppm/°C).38 The microwave dielectric properties of MGO and ZGO ceramics with varying CaTiO3 content are presented in Table II. The τf can be adjusted to a near-zero value with the addition of 8 mol% CaTiO3: −2 ppm/°C for MGO and −4 ppm/°C for ZGO ceramics. As shown in Fig. 8a and b, the XRD diffraction peaks of 0.92 MGO–0.08 CaTiO3 and 0.92 ZGO–0.08 CaTiO3 can be well indexed by the JCPDS cards for MGO, ZGO, and CaTiO3, indicating that CaTiO3 does not react with MGO and ZGO ceramics. Table III provides a comparison of the microwave dielectric properties of spinel-structured gallate ceramics prepared through the conventional two-step solid-state sintering route and the reaction sintering method employed in this work. The microwave dielectric properties of MGO and ZGO ceramics prepared by reaction sintering are comparable to those fabricated via the conventional solid-state method. This suggests that MGO and ZGO ceramics are suitable for preparation by reaction sintering, offering a cost advantage.
Conclusions
In conclusion, MgGa2O4 and ZnGa2O4 ceramics were successfully prepared by a simple reaction sintering method. The crystal structure, microstructure, and microwave dielectric properties of MgGa2O4 and ZnGa2O4 ceramics were investigated. Based on the analysis of the structure and performance, the arrangement of cations in MgGa2O4 ceramics will affect the structural parameters and microwave dielectric properties. The volatilization of zinc elements and abnormal grain growth can degrade the microstructure of ZnGa2O4 ceramics and further deteriorate the performance. Excellent microwave dielectric properties were obtained: εr = 9.4, Q×f = 167,500 GHz, and τf = −63 ppm/°C for MgGa2O4 ceramics sintered at 1610°C; εr = 10.5, Q×f = 126,900 GHz, and τf = −60 ppm/°C for ZnGa2O4 ceramics sintered at 1550°C. Through the incorporation of CaTiO3, the τf values of MgGa2O4 and ZnGa2O4 ceramics were adjusted to near-zero levels, resulting in overall improved properties: εr = 11.4, Q×f = 121,000 GHz, and τf = −2 ppm/°C for 0.92 MgGa2O4–0.08 CaTiO3 ceramics; εr = 12.3, Q×f = 92,000 GHz, and τf = −4 ppm/°C for 0.92 ZnGa2O4–0.08 CaTiO3 ceramics. These results indicate that MgGa2O4 and ZnGa2O4 ceramics prepared by reaction sintering are promising candidates for use in electronic devices and 5G communication applications.
References
D.S. McClure, The distribution of transition metal cations in spinels. J. Phys. Chem. Solids 3, 311 (1957).
T. Parida, A. Karati, K. Guruvidyathri, B.S. Murty, and G. Markandeyulu, Novel rare-earth and transition metal-based entropy stabilized oxides with spinel structure. Scr. Mater. 178, 513 (2020).
A.K. Lebechi, A.K. Ipadeola, K. Eid, A.M. Abdullah, and K.I. Ozoemena, Porous spinel-type transition metal oxide nanostructures as emergent electrocatalysts for oxygen reduction reactions. Nanoscale 14, 10717 (2022).
P.G. Casado, and I. Rasines, Crystal data for the spinels MGa2O4 (M = Mg, Mn). Z. Für Krist. 160, 33 (1982).
A. Kan, T. Moriyama, S. Takahashi, and H. Ogawa, Cation distributions and microwave dielectric properties of spinel-structured MgGa2O4 ceramics. Jpn. J. Appl. Phys. 52, 09KH01 (2013).
H. Ouchi and S. Kawashima, Dielectric ceramics for microwave application. Jpn. J. Appl. Phys. 24, 60 (1985).
I.M. Reaney and D. Iddles, Microwave dielectric ceramics for resonators and filters in mobile phone networks. J. Am. Ceram. Soc. 89, 2063 (2006).
R.D. Shannon and G.R. Rossman, Dielectric constant of MgAl2O4 spinel and the oxide additivity rule. J. Phys. Chem. Solids 52, 1055 (1991).
K.P. Surendran, P.V. Bijumon, P. Mohanan, and M.T. Sebastian, (1–x)MgAl2O4-xTiO2 dielectrics for microwave and millimeter wave applications. Appl. Phys. A 81, 823 (2005).
K.P. Surendran, N. Santha, P. Mohanan, and M.T. Sebastian, Temperature stable low loss ceramic dielectrics in (1–x)ZnAl2O4-xTiO2 system for microwave substrate applications. Eur. Phys. J. B Condens. Matter Complex Syst. 41, 301 (2004).
C.W. Zheng, S.Y. Wu, X.M. Chen, and K.X. Song, Modification of MgAl2O4 microwave dielectric ceramics by Zn substitution. J. Am. Ceram. Soc. 90, 1483 (2007).
C.-L. Huang, C.-Y. Tai, C.-Y. Huang, and Y.-H. Chien, Low-loss microwave dielectrics in the spinel-structured (Mg1−xNix)Al2O4 solid solutions. J. Am. Ceram. Soc. 93, 1999 (2010).
S. Wu, J. Xue, and Y. Fan, Spinel Mg(Al, Ga)2O4 solid solution as high-performance microwave dielectric ceramics. J. Am. Ceram. Soc. 97, 3555 (2014).
J. Yu, C. Shen, and T. Qiu, Effect of microwave sintering on the microstructure and dielectric properties of 0.92MgAl2O4–0.08(Ca0.8Sr0.2)TiO3 Ceramics. J. Mater. Sci. Mater. Electron. 26, 2737 (2015).
W. Lei, W.-Z. Lu, D. Liu, and J.-H. Zhu, Phase evolution and microwave dielectric properties of (1–x)ZnAl2O4−xMg2TiO4 ceramics. J. Am. Ceram. Soc. 92, 105 (2009).
X. Lu, Z. Du, B. Quan, W. Bian, H. Zhu, and Q. Zhang, structural dependence of the microwave dielectric properties of Cr3+-substituted ZnGa2O4 spinel ceramics: crystal distortion and vibration mode studies. J. Mater. Chem. C 7, 8261 (2019).
J. Xue, S. Wu, and J. Li, Synthesis, microstructure, and microwave dielectric properties of spinel ZnGa2O4 ceramics. J. Am. Ceram. Soc. 96, 2481 (2013).
A. Kan, S. Takahashi, T. Moriyama, and H. Ogawa, Influence of Zn substitution for Mg on microwave dielectric properties of spinel-structured (Mg1−xZnx)Ga2O4 solid solutions. Jpn. J. Appl. Phys. 53, 09PB03 (2014).
X. Lu, W. Bian, Y. Li, H. Zhu, Z. Fu, and Q. Zhang, Cation distributions and microwave dielectric properties of Cu-substituted ZnGa2O4 spinel ceramics. Ceram. Int. 43, 13839 (2017).
X. Lyu, L. Ren, L. Xin, Z. Li, X. Zhang, Z. Luo, P. Shao, Y. Zhang, P. Lyu, and M. Zhang, Low temperature sintering, structure and microwave dielectric properties of novel (1–x)MgGa2O4–xCuMoO4 ceramics for LTCC applications. ACS Sustain. Chem. Eng. 11, 7002 (2023).
Y. Xiong, H. Xie, Z. Rao, L. Liu, Z. Wang, and C. Li, Compositional modulation in ZnGa2O4 via Zn2+/Ge4+ Co-doping to simultaneously lower sintering temperature and improve microwave dielectric properties. J. Adv. Ceram. 10, 1360 (2021).
Y.-C. Liou, C.-T. Wu, K.-H. Tseng, and T.-C. Chung, Synthesis of BaTi4O9 ceramics by reaction-sintering process. Mater. Res. Bull. 40, 1483 (2005).
L. He, H. Yu, M. Zeng, E. Li, J. Liu, and S. Zhang, Phase compositions and microwave dielectric properties of MgTiO3-based ceramics obtained by reaction-sintering method. J. Electroceramics 40, 360 (2018).
X. Wang, K. Liu, S. Zhou, S. He, S. Deng, Y. Xiao, X. Chen, and H. Zhou, Reaction sintering and mechanism of microwave dielectric ceramic with K2NiF4 structure and perovskite structure. J. Mater. Sci. Mater. Electron. 33, 2213 (2022).
B.W. Hakki and P.D. Coleman, A dielectric resonator method of measuring inductive capacities in the millimeter range. IRE Trans. Microw. Theory Tech. 8, 402 (1960).
A. Luchechko, V. Vasyltsiv, M. Kushlyk, D. Slobodzyan, M. Baláž, J. Cebulski, K. Szmuc, J. Szlęzak, and Y. Shpotyuk, Structural and luminescence characterization of β-Ga2O3 nanopowders obtained via high-energy ball milling. Appl. Nanosci. 13, 5149 (2023).
I.D. Brown and R.D. Shannon, Empirical bond-strength–bond-length curves for oxides. Acta Crystallogr. A 29, 266 (1973).
I.D. Brown and K.K. Wu, Empirical parameters for calculating cation-oxygen bond valences. Acta Crystallogr. B 32, 1957 (1976).
R. Shi, S. Zhu, R. Muhammad, T. Zhou, B. Liu, M. Mao, D. Wang, and K. Song, Degree of inversion of A/B lattice sites and microwave/millimeter wave/terahertz dielectric properties of MgAl2-x(Zn0.5Mn0.5)xO4 ceramics. J. Eur. Ceram. Soc. 43, 3324 (2023).
W.B. White and B.A. DeAngelis, Interpretation of the vibrational spectra of spinels. Spectrochim. Acta Part Mol. Spectrosc. 23, 985 (1967).
V. D’Ippolito, G.B. Andreozzi, D. Bersani, and P.P. Lottici, Raman fingerprint of chromate, aluminate and ferrite spinels. J. Raman Spectrosc. 46, 1255 (2015).
D. Dohy, G. Lucazeau, and A. Revcolevschi, Raman spectra and valence force field of single-crystalline β Ga2O3. J. Solid State Chem. 45, 180 (1982).
R. Rao, A.M. Rao, B. Xu, J. Dong, S. Sharma, and M.K. Sunkara, Blueshifted raman scattering and its correlation with the [110] growth direction in gallium oxide nanowires. J. Appl. Phys. 98, 094312 (2005).
N.A. Gribchenkova, A.V. Steblevsky, and A.S. Alikhanyan, vaporization in the Ga2O3−ZnO system by high temperature mass spectrometry. J. Chem. Thermodyn. 115, 1 (2017).
S. Liu, H. Li, R. Xiang, P. Zhang, X. Chen, Q. Wen, and H. Hu, Effect of substituting Al3+ for Ti4+on the microwave dielectric performance of Mg2Ti1-xAl4/3xO4 (00.1 ≤ x ≤ 0.09) ceramics. Ceram. Int. 47, 33064 (2021).
M. Zhou, H. Chen, Y. He, G. Zhou, X. Zhang, S. Zhang, and B. Tang, Phase composition, crystal structure, and microwave dielectric properties of Nb-doped and Y-deficient yttrium aluminum garnet ceramics. J. Eur. Ceram. Soc. 42, 5705 (2022).
L. Zhang, H. Ren, H. Peng, H. Lin, and Q. Yang, Sintering behavior of 0.95MgTiO3-0.05CaTiO3 ceramics with high densification, high Q and enhanced mechanical properties for 5G massive MIMO technology: effect of particle gradation. Ceram. Int. 50, 4462 (2024).
K. Wakino, Recent development of dielectric resonator materials and filters in Japan. Ferroelectrics 91, 69 (1989).
S. Wu, J. Xue, R. Wang, and J. Li, Synthesis, characterization and microwave dielectric properties of spinel MgGa2O4 ceramic materials. J. Alloys Comp. 585, 542 (2014).
X. Lu, B. Quan, K. Zheng, P. Chu, J. Wang, G. Shen, Q. Zhang, and F. Xu, Sc modification induced short-range cation ordering and high microwave dielectric performance in ZnGa2O4 spinel ceramics. J. Alloys Comp. 873, 159758 (2021).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 62171080 and 52102123) and Opening Project of State Key Laboratory of High Performance Ceramics and Superfine Microstructure (SKL202203SIC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Z., Li, S., Zheng, Z. et al. Crystal Structure, Microstructure, and Microwave Dielectric Properties of MgGa2O4 and ZnGa2O4 Ceramics Prepared by a Reaction Sintering Method. J. Electron. Mater. 53, 2240–2249 (2024). https://doi.org/10.1007/s11664-024-10928-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10928-x