Abstract
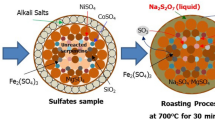
The sulfation roasting leaching process has been investigated as an alternative method for recovering nickel and cobalt from laterite ore. This process involves converting the nickel and cobalt in the ore into sulfates, which can then be dissolved through water leaching, while the iron remains as an insoluble oxide. However, the presence of other components such as magnesium oxide and silica in the laterite ore may hinder the efficiency of the extraction process. This study aimed to examine the influence of silica and magnesium oxide in three different types of laterite ore on the extraction of nickel and cobalt through the sulfation roasting leaching process. Various characterization techniques, including thermodynamic estimation, X-ray diffraction, and chemical analyses by ICP, were employed to analyze the ores, leached solutions, and solid residues. Phase quantification using the Rietveld method through TOPAZ analysis software was conducted on the original ore, sulfation roasted ore, and solid residue. The findings revealed that the recovery of nickel and cobalt was significantly influenced by the silica and magnesium oxide content in the original ore. The ore with the lowest silica and magnesium oxide content exhibited the highest recovery rates, with nickel recovery reaching 86.3 pct and cobalt recovery reaching 85.7 pct. Conversely, the serpentine-rich ore demonstrated the lowest recovery rates, with nickel recovery at 69.9 pct and cobalt recovery at 68.0 pct. The mechanism of how silica and magnesium oxide affect the extraction of nickel and cobalt by sulfation roasting leaching process was clarified.

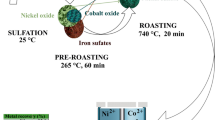
(Adapted from Ref. [15])















Similar content being viewed by others
References
M.A. Rhamdhani, P.C. Hayes, and E. Jak: Miner. Proc. Extract Met. Trans. Inst. Min. Met. Sect. C, 2009, vol. 118, pp. 129–45.
P. Meshram, B. Abhilash, and D. Pandey: Min. Proc. Extract. Met. Rev., 2019, vol. 40, pp. 157–93.
F.K. Crundwell, M.S. Moats, V. Ramachandran, T.G. Robinson, and W.G. Davenport: Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier, Amsterdam, 2011, pp. 21–37.
A.D. Dalvi, W.G. Bacon, and R.C. Osborne: PDAC 2004 international conference trade show and investors exchange. Toronto, 2004, vol. 2004, pp. 1–27.
T. Norgate and S. Jahanshahi: Miner. Eng., 2010, vol. 23, pp. 65–73.
P.P.M. Ribeiro, L.C.M. de Souza, R. Neumann, I.D.D. Santos, and A.J.B. Dutra: J. Mater. Res. Technol., 2020, vol. 9, pp. 12404–15.
B.B. Kar and Y.V. Swamy: Miner. Eng., 2000, vol. 13, pp. 1635–40.
Y. Xu, Y. Xie, L. Yan, and R. Yang: Hydrometallurgy, 2005, vol. 80, pp. 280–85.
Y.V. Swamy, B.B. Kar, and J.K. Mohanty: Hydrometallurgy, 2003, vol. 69, pp. 89–98.
H. Basturkcu and N. Acarkan: Physicochem. Probl. Miner. Process., 2016, vol. 52, pp. 564–74.
X. Guo, D. Li, K.H. Park, Q. Tian, and Z. Wu: Hydrometallurgy, 2009, vol. 99, pp. 144–50.
H.H. Emons, G. Ziegenbalg, R. Naumann, and F. Paulik: J. Therm. Anal., 1990, vol. 36, pp. 1265–79.
P.P.M. Ribeiro, I.D.D. Santos, R. Neumann, A. Fernandes, and A.J.B. Dutra: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 1739–54.
M. Porus, C. Labbez, P. Maroni, and M. Borkovec: J. Chem. Phys., 2011, vol. 135, p. 064701.
A. Oxley and N. Barcza: Miner. Eng., 2013, vol. 54, pp. 2–13.
R. Neumann, A.N. Avelar, and G.M. da Costa: Miner. Eng., 2014, vol. 55, pp. 80–86.
L. Wei, F. Qiming, O. Leming, Z. Guofan, and L. Yiping: Hydrometallurgy, 2009, vol. 96, pp. 171–75.
A. Ammasi: Trans. Indian Inst. Met., 2020, vol. 73, pp. 93–98.
P.P.M. Ribeiro, R. Neumann, I.D.D. Santos, M.C. Rezende, P. Radino-Rouse, and A.J.B. Dutra: Miner. Eng., 2019, vol. 131, pp. 90–97.
K. Liu, Q. Chen, and H. Hu: Hydrometallurgy, 2009, vol. 98, pp. 281–86.
M.N. Scheidema and P. Taskinen: Ind. Eng. Chem. Res., 2011, vol. 50, pp. 9550–56.
E.A. Abdel-Aal and M.M. Rashad: Hydrometallurgy, 2004, vol. 74, pp. 189–94.
R.C. Hubli, J. Mittra, and A.K. Suri: Hydrometallurgy, 1997, vol. 44, pp. 125–34.
J.M. Skeaff and A.W. Espelund: Can. Metall. Q., 1973, vol. 12, pp. 445–54.
R.K.S. Hariyanto, L.T. Da Rocha, S.J. Kim, and S.M. Jung: Metall. Mater. Trans. B, 2021, vol. 54B, pp. 2915–28.
M.A.R. Önal, C.R. Borra, M. Guo, B. Blanpain, and T. van Gerven: J. Sustain. Met., 2015, vol. 1, pp. 199–215.
H. Tagawa: Thermochim. Acta, 1984, vol. 80, pp. 23–33.
V.S. Ranjani, A.P. James, P.F. Edward, S. Ming-Shing, and L.M. Angela: Appl. Surf. Sci., 1999, vol. 152, pp. 219–36.
C. Li, J. Zhao, and Y. Zhan: Nanomaterials, 2021, vol. 11, p. 2314.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hariyanto, R.K.S., Tomas da Rocha, L., Cho, SK. et al. Influence of SiO2 and MgO on Extraction of Nickel and Cobalt from Laterite Ores by Sulfation Roasting Leaching Process. Metall Mater Trans B (2024). https://doi.org/10.1007/s11663-024-03039-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11663-024-03039-9