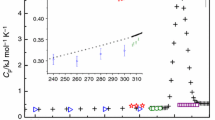
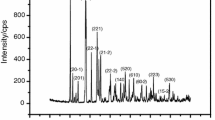
Abstract
In the course of thermal decomposition of the magnesium sulphate hydrates MgSO4.nH2O (n=7; 6; 5; 4; 3; 2; 5/4; 1) the intermediate steps MgSO4.3H2O, MgSO4.2H2O, MgSO4.H2O andΒ — MgSO4 are observed under quasi-isothermal and quasi-isobaric conditions atp ≅ 0.1 MPa dependent on the water contents. The structure of the obtained monohydrate phase is identical with that of kieserite. Thermal decomposition of the magnesium sulphate hydrates is essentially influenced by the water vapour partial pressure.
Zusammenfassung
Beim thermischen Abbau der Magnesiumsulfathydrate MgSO4.nH2O (n=7; 6; 5; 4; 3; 2; 5/4; 1) unter quasiisothermen- und quasiisobaren Bedingungen werden bei p−0.1 MPa, in AbhÄngigkeit von den Wassergehalten, die Zwischenstufen MgSO4.3H2O; MgSO4.2H2O; MgSO4.H2O sowieΒ-MgSO4 beobachtet. Die erhaltene Monohydratphase ist strukturell mit Kieserit identisch. Der thermische Abbau der Magnesiumsulfathydrate wird wesentlich vom Wasserdampfpartialdruck beeinflu\t.
Similar content being viewed by others
References
R. Robson, J. Am. Chem. Soc. 49 (1927) 2772.
R. Hodenberg, R. Kühn, Kali und Steinsalz, 4 (1967) 326.
Gmelin, Handbuch der anorg. Chemie; Magnesium, Teil B Berlin 1939.
M. Polo, N. Gérard and M. Lallemant, C. R. Acad. Sci. Paris, t. 272 Série C; (1971) 642.
L. G. Berg and M. T. Saibova, Z. Neorg. Chim., 7 (1962) 91.
S. Hamad, Thermochim. Acta, 13 (1975) 409.
A. S. Phadnis, Thermochim. Acta, 43 (1981) 249.
Zivkovic, Rud. Mt. Zb. Ljubljana, 29 (1982) 189.
K. Heide, Chemie der Erde 24 (1965) 279.
M. Lallemant and G. Watelle-Marion, C. R. Acad. Sc. Paris, t. 264 Série C; (1967) 2030.
M. Lallemant and G. Watelle-Marion, C. R. Acad. Sc. Paris, t. 265, Série C; (1967) 627.
J. Paulik, F. Paulik and M. Arnold, Thermochim. Acta, 50 (1981) 105.
J. Paulik and F. Paulik, Comprehensive Analytical Chemistry, G. Svehla (Ed.) Vol. XII, Part A, Elsevier, Amsterdam. 1981.
F. Winkler, Dissertation, TH Leuna-Merseburg 1968.
H. H. Emons, T. Pohl, R. Naumann and H. Voigt, Thermische Analysenverfahren in Industrie und Forschung, Vol. 3, FSU Jena, 1985, p. 65.
R. Müller, and H. Schübl, Freiberger Forschungshefte A 683, p. 78.
H. J. Rösler, Salzmikroskopie, Lehrbrief der Bergakademie Freiberg, 1968.
M. Lallemant and. G. Watelle-Marion, Compt. Rend., 265 c (1967) 627.
Author information
Authors and Affiliations
Additional information
We gratefully acknowledge the assistance of Dr. K. Köhnke in providing X-ray diffraction investigations and the scanning electron micrographs provided by Dr. Ulrich, Bergakademie Freiberg.
Rights and permissions
About this article
Cite this article
Emons, H.H., Ziegenbalg, G., Naumann, R. et al. Thermal decomposition of the magnesium sulphate hydrates under quasi-isothermal and quasi-isobaric conditions. Journal of Thermal Analysis 36, 1265–1279 (1990). https://doi.org/10.1007/BF01914050
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01914050