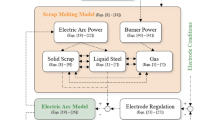
Abstract
The scrap preheating stage performed before the main scrap melting stage has a growing significance in the modern electric arc furnace (EAF), which utilizes the coherent jet burners to preheat and melt the scrap to improve the overall energy efficiency within the furnace, but the corresponding computational fluid dynamics (CFD) modeling and analysis of this process were still very limited. The present paper developed a fully integrated and self-consistent CFD model to simulate the multi-physics during the burner preheating and melting process in a full-size industrial EAF, including combustion flame, interphase heat transfer, scrap melting, and liquid steel re-solidification. The innovative dual-cell approach and stack approach were proposed to simulate the porous scrap preheating/melting and its dynamic collapse process. The immersed scrap preheating experiment was first time designed and implemented in an industrial EAF for model validation with an average difference of 12.7 pct. The model was used to gain fundamental insights into the scrap preheating stage and evaluate the effects of different factors on the burner preheating and melting characteristics. The guidance was provided to achieve optimal operational efficiency and energy utilization in the industrial furnace.
















Similar content being viewed by others
Change history
14 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11663-023-02831-3
Abbreviations
- p :
-
Phase subscript
- q :
-
Phase subscript
- i:
-
Specie subscript
- g:
-
Gas phase
- l:
-
Liquid phase
- s:
-
Solid phase
- m:
-
Mixture
- \({{C}_{\text{D}}}^{{\prime}}\) :
-
Constant
- \({\overrightarrow{F}}_{\text{df}}\) :
-
Drag force
- \({\overline{\overline{Q}}}_{\text{s}}\) :
-
Volumetric energy exchange
- \({\dot{m}}_{\text{s,mt}}\) :
-
Solid mass transfer rate
- \({h}_{\text{fusion}}\) :
-
Latent heat of fusion
- \({h}_{\text{v}}\) :
-
Volumetric heat transfer coefficient
- \({C}_{1}\) :
-
Constant
- \({C}_{1\varepsilon }\) :
-
Constant
- \({C}_{2}\) :
-
Constant
- \({C}_{2\varepsilon }\) :
-
Constant
- \({C}_{\text{D}}\) :
-
Drag coefficient
- \({C}_{\text{T}}\) :
-
Function dependent on gas temperature and velocity field
- \({C}_{\text{p,eff}}\) :
-
Effective specific heat capacity
- \({C}_{\text{p}}\) :
-
Specific heat capacity
- \({C}_{\text{s}}\) :
-
Scrap inertial resistance factor
- \({D}_{\text{T}}\) :
-
Thermal Soret diffusion coefficient
- \({E}_{\text{s}}\) :
-
Solid energy
- \({G}_{\text{k}}\) :
-
Kinetic energy produced by turbulence
- \({K}_{\text{eff}}\) :
-
Effective thermal conductivity
- \({M}_{\text{w}}\) :
-
Molecular weight
- \({M}_{\tau }\) :
-
Turbulent Mach number
- \({M}_{\tau 0}\) :
-
Constant
- \({Q}_{\text{ht}}\) :
-
Interphase heat transfer
- \({Sc}_{\text{t}}\) :
-
Turbulent Schmidt number
- \({T}_{\text{liquidus}}\) :
-
Liquidus temperature
- \({T}_{\text{solidus}}\) :
-
Solidus temperature
- \({T}_{\text{t}}\) :
-
Local total temperature
- \({V}_{\text{c}}\) :
-
Cell volume
- \({V}_{\text{pit}}\) :
-
Melting pit volume
- \({a}_{\text{g}}\) :
-
As acoustic velocity
- \({a}_{\varepsilon }\) :
-
Emissivity weighting factor
- \({b}_{\varepsilon }\) :
-
Emissivity gas temperature polynomial coefficients
- \(\overrightarrow{g}\) :
-
Gravity
- \(\overrightarrow{j}\) :
-
Mass diffusion
- \({k}^{{\prime}}\) :
-
Absorption coefficient
- \({m}_{\text{s}}\) :
-
Solid mass
- \({n}^{{\prime}}\) :
-
Refractive index
- \(\overrightarrow{r}\) :
-
Beam position
- \(\overrightarrow{s}\) :
-
Beam direction
- \(\overrightarrow{v}\) :
-
Velocity vector
- \({v}^{{\prime}}\) :
-
Stoichiometric coefficient for reactant
- \({v}^{{\prime\prime} }\) :
-
Stoichiometric coefficient for product
- \({\Omega }^{{\prime}}\) :
-
Solid angle
- \({\beta }_{\text{s}}\) :
-
Scrap permeability
- \({\gamma }_{\text{s}}\) :
-
Scrap porosity
- \({\varepsilon}^{{\prime}}\) :
-
Total emissivity
- \({\mu }_{\text{t}}\) :
-
Turbulent viscosity
- \({\sigma }_{k}\) :
-
Constant
- \({\sigma }_{\text{s}}\) :
-
Scattering coefficient
- \({\sigma }_{\varepsilon }\) :
-
Constant
- \(\overline{\overline{\tau }}\) :
-
Stress–strain tensor
- \({h}_{\text{c}}\) :
-
Cell layer height
- \({h}_{pq}\) :
-
Interphase heat transfer coefficient
- h :
-
Sensible enthalpy
- A :
-
Empirical constant
- \({A}_{pq}\) :
-
Interfacial contact area
- B :
-
Empirical constant
- D :
-
Mass diffusion coefficient
- E :
-
Energy
- F :
-
Factor layer matrix
- G :
-
Extreme difference
- \(H(x)\) :
-
Heaviside function
- I :
-
Radiation intensity
- K :
-
Average value of given evaluation index
- L :
-
Level layer matrix
- M :
-
Experimental evaluation index layer matrix
- Pr :
-
Prandtl number
- R :
-
Net rate of chemical reaction production
- Re :
-
Reynolds number
- S :
-
Modulus of the mean rate-of-strain tensor
- T :
-
Temperature
- Y :
-
Local mass fraction
- a :
-
Absorption coefficient
- d :
-
Characteristic diameter
- \(f\left({M}_{\tau }\right)\) :
-
Function dependent on Mach number
- \(f\left(\omega \right)\) :
-
Coefficient dependent on material porosity
- k :
-
Turbulent kinetic energy
- p :
-
Pressure
- s :
-
Path length
- t :
-
Flow time
- \(\alpha \) :
-
Volume fraction
- \(\varepsilon \) :
-
Turbulent dissipation rate
- \(\lambda \) :
-
Thermal conductivity
- \(\mu \) :
-
Molecular viscosity
- \(\xi \) :
-
Constant
- \(\rho \) :
-
Density
- \(\sigma \) :
-
Stefan–Boltzmann constant
- \(\omega \) :
-
Weight matrix
- \(\phi \) :
-
Phase function
References
B.E. Hites: Recycling Today, https://www.recyclingtoday.com/article/the-growth-of-eaf-steelmaking/, accessed 30 April 2022.
M.J. Thomson, E.J. Evenson, M.J. Kempe, and H.D. Goodfellow: Ironmak. Steelmak., 2000, vol. 27, pp. 273–79.
S&P Global Commodity Insights: US Steel Sector Thrives as Mills Move up Quality Ladder, https://www.spglobal.com/commodityinsights/en/market-insights/blogs/, accessed 09 May 2022.
AISTech: 2019 AIST Industry Roundups, http://digital.library.aist.org/pages/PR-RU2019-2.htm/, accessed June 7, 2022.
E. Pretorius, H. Oltmann, and J. Jones: EAF Fundamentals, LWB Refractories, New York, 2010, pp. 1–54.
M. Alam, J. Naser, G. Brooks, and A. Fontana: Metall. Mater. Trans. B., 2010, vol. 41B, pp. 1354–67.
F. Liu, R. Zhu, K. Dong, and S. Hu: Metall. Mater. Trans. B., 2016, vol. 47B, pp. 228–43.
G. Wei, R. Zhu, X. Wu, L. Yang, K. Dong, T. Cheng, and T. Tang: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 1405–20.
Y. Chen, A.K. Silaen, and C.Q. Zhou: Processes, 2020, vol. 8, p. 700.
J.D. Hernandez, L. Onofi, and S. Engell: IFAC Papers OnLine, 2019, vol. 52, pp. 30–35.
Y. Chen, S. Ryan, A.K. Silaen, and C.Q. Zhou: Metall. Mater. Trans. B., 2022, vol. 53B, pp. 1–20.
V. Logar, D. Dovžan, and I. Škrjanc: ISIJ Int., 2012, vol. 52, pp. 402–12.
F. Opitz and P. Treffinger: Metall. Mater. Trans. B., 2016, vol. 47B, pp. 1489–1503.
A, Fathi, Y. Saboohi, I. Škrjanc, and V. Logar: Steel Res. int., 2017, vol. 88, p. 1600083.
K. Mandal and G.A. Irons: Metall. Mater. Trans. B., 2013, vol. 44B, pp. 184–95.
K. Mandal and G.A. Irons: Metall. Mater. Trans. B., 2013, vol. 44B, pp. 196–209.
K. Mandal: PhD dissertation, 2010.
Y. Chen, Q. Luo, S. Ryan, N. Busa, A.K. Silaen, and C.Q. Zhou: Appl. Therm. Eng., 2022, vol. 212, 118596.
D. Guo and G.A. Irons: Appl. Math. Model., 2008, vol. 32, pp. 2041–49.
Y. Chen, Q. Luo, A.K. Silaen, and C.Q. Zhou: Front. Mater., 2020, vol. 7, pp. 336–51.
S. Ergun: Chem. Eng. Prog., 1952, vol. 48, pp. 89–94.
N. Kladias and V. Prasad: J. Thermophys. Heat Transf., 1991, vol. 5, pp. 560–76.
L. Schiller: Zeit. Ver. Deutsch. Ing., 1933, vol. 77, pp. 318–20.
X. Zhang, R. Takahashi, H. Nogami, and J. Yagi: Tetsu-to-Hagané, 2001, vol. 87, pp. 410–17.
W.E. Ranz and W.R. Marshall: Chem. Eng. Prog., 1952, vol. 48, pp. 141–46.
R.W. Lewis and K. Ravindran: Int. J. Numer. Methods Eng., 2000, vol. 47, pp. 29–59.
N. Wakao, S. Kaguei, and T. Funazkri: Chem. Eng. Sci., 1979, vol. 34, pp. 325–36.
C. C. Furnas: U.S. Bureau of Mines Bulletin, 1932, p. 261.
B.I. Kitaev, Y.G. Yaroshenko, and V.D. Suchkov: Heat Exchange in Shaft Furnaces, 1st ed. Pergamon Press, Oxford, 1967.
S. Saravanan, G. Nagarajan, and S. Sampath: Open J. Optim., 2012, vol. 1, pp. 25–33.
Acknowledgments
The authors would like to thank the Steel Manufacturing Simulation and Visualization Consortium (SMSVC) members for funding this project. The NLMK Indiana and the Center for Innovation through Visualization and Simulation (CIVS) at Purdue University Northwest are also gratefully acknowledged for providing all the resources for this work. The authors also appreciate the great help from Eugene Pretorius (NUCOR), Jianghua Li (Cleveland-Cliffs), Yufeng Wang (SSAB), Joe Maiolo (Linde), and Hamzah Alshawarghi (Linde).
Conflict of interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Contributions
Conceptualization, YC; methodology, YC; model development, YC; model validation, YC; parametric studies, YC; data post processing, YC; experiment, SR; manuscript draft, YC; review and editing, SR, AKS, and YC; project supervision, CQZ; and funding acquisition, CQZ.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
The experimental evaluation index layer matrix (M1): assuming there are i factors in total for the optimization and each factor has m levels, the average value of the evaluation index at the j level of factor \({A}_{i}\) is denoted as \({K}_{ij}\). In the present study, the larger the evaluation index, the better the burner preheating and melting results, thus establishing the matrix \({M}_{1}\) as follows:
The factor layer matrix (\({F}_{1}\)): the elements in the matrix can be then determined through \(1/\sum_{j=1}^{m}{K}_{ij}\) from the values in \({M}_{1}\):
The level layer matrix (\({L}_{1}\)): the elements in the matrix can be then determined through \({G}_{i}/\sum_{i=1}^{i}{G}_{ij}\) based on the range analysis results to compute the ratio of the extreme difference of factor \({A}_{i}\) to the total of all extreme differences:
The weight matrix (\({\omega }_{1}\)): the weights of three factors within all three levels can be determined by multiplying three matrixes above:
For the evaluations of scrap porosity and initial scrap temperature, the same procedure can be performed to calculate \({\omega }_{2}\) and \({\omega }_{3}\):
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Ryan, S., Silaen, A.K. et al. An Investigation into EAF Burner Preheating and Melting Characteristics: CFD Model Development and Experimental Validation. Metall Mater Trans B 54, 1068–1087 (2023). https://doi.org/10.1007/s11663-023-02741-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02741-4