Abstract
The prereduction reactions in the submerged arc furnace (SAF) are highly decisive of the total carbon, and energy consumption of the ferromanganese process. Therefore, understanding the dependency of prereduction behavior on ore characteristics and furnace conditions is of paramount importance. Prereduction behavior of Comilog, Nchwaning and UMK ores with solid carbon was studied in an open 75 kVA induction furnace set up simulating the conditions of an industrial SAF. In addition, the ores were thermogravimetrically studied with non-isothermal heating to 1000 °C in a flowing 70/30 CO/CO2 atmosphere. Gasification of carbon according to Boudouard reaction was considered significant for carbonate ores in induction furnace setup. UMK had a greater extent of prereduction compared to Nchwaning which is low in carbonates and Comilog which is known to have a higher CO reactivity at similar CO contents. TGA rate curves shows that prereduction of UMK ore occurred with two major distinctive steps. The first one was a combination of prereduction reactions and decomposition of carbonates with a peak temperature of 700 °C, and the second peak was the decomposition of calcite at 900 °C. The O/Mn ratio for UMK shows that prereduction is already completed prior to the second decomposition peak at 900 °C. In addition to prereduction reactions, Nchwaning is also characterized by an endothermic step which is decomposition of carbonates at 900 °C. Comilog ore produced the most fines followed by Nchwaning and lastly UMK on decrepitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ferromanganese (FeMn) alloys are mainly produced in the submerged arc furnace (SAF) with a constant supply of raw materials such as manganese ore, sinter, metallurgical coke, and flux blended in predetermined ratios[1]. The FeMn production process is energy intensive with requirements ranging between 2.0 and 3.5 MWh per tonne alloy produced.[1,2,3,4] Energy for the process is supplied by conversion of electrical energy into heat at the electrode tip through resistive heating of the coke bed to attain smelting temperatures. A temperature gradient develops in the furnace through heat exchange between descending burden materials and hot ascending CO gas generated from the smelting reactions. The furnace is typically divided into two main zones namely the prereduction zone (200 °C to 1200 °C) and the coke bed zone (1200 °C to 1600 °C). Initially, iron oxides and higher manganese oxides (MnO2, Mn2O3 and Mn3O4) contained in manganese ores are prereduced in the prereduction zone by CO gas to metallic Fe and MnO. Subsequently, the MnO is reduced in the coke bed zone from liquid slag to FeMn metal by means of solid carbon.[1,5] The typical ferromanganese process reactions and their corresponding standard reaction enthalpies calculated using HSC Chemistry® software[6] are presented as follows:
The solid–gas prereduction reactions reactions [1] through [5] are mostly exothermic (apart from reaction [5]) and produce CO2. Carbonates in the charge mixture typically consist of Ca, Mg and Mn bearing carbonates or dolomite which decompose to produce CO2. The resultant CO2 gas from prereduction of Mn3O4 and decomposition of carbonates, will react with solid carbon according to the highly endothermic Boudouard reaction (reaction [7]) at temperatures greater than 800 °C, thereby increasing the consumption of energy and carbon. In the coke bed zone, the energy requirements and necessary quantity of carbon to produce FeMn alloy by direct reduction (reaction [8]) of the prereduced ore is fixed for a specific alloy composition. As such, the total carbon and energy consumption is highly dependent on the extent of gaseous reduction of higher manganese oxides in the prereduction zone and the extent of the Boudouard reaction. Therefore, the gas–solid reactions in the prereduction zone are to a large extent decisive for the variations of both coke and energy in ferromanganese production.[7] This will also affect the total CO2 emissions from the process.
Characteristics of the prereduction process differ and are significantly dependent on the ore type used. This is mainly because manganese ores used by industry in the production of FeMn alloys have different origins and therefore differ in mineralogy, chemical and physical properties. Typically, metallurgical grade ores contain more than 35 pct Mn and literature[8,9,10,11,12,13,14] covers properties of ores with Comilog, CVRD, Groote Eylandt, Wessels and Mamatwan type ores as examples. Several studies[10,11,15,16,17,18,19,20,21,22] have been conducted on the prereduction of manganese ores by either CO gas or solid carbon.
Kor[15] studied the rate of reduction of Mn3O4 by CO and carbonaceous reductants namely; coconut charcoal, coke and pure graphite in the temperature range 900 °C to 1200 °C. The rate of reduction in CO was determined to be fast and the overall rate of reduction by a carbonaceous reductant was influenced by the oxidation of the carbon by CO2. The oxidation of carbon by CO2 was found to be significantly pronounced when the reductant was either carbon or coke and in addition, catalyzed by Mn3O4. Halim et al.[16] studied the prereduction reactions for a 57.2 pct MnO2 ore in a rotary kiln at 800 °C to 1100 °C. They used large sample sizes (3 kg) with particle sizes (100 to 400 μm) mixed with Pet Coke. The coke (carbon) was mixed at 1.25 times the stoichiometric ratio for reducing iron and manganese oxides. The crucible was kept in an inert atmosphere of argon during the experiment. Micro TGA was also used to measure weight loss for 1 g of pelletized samples, also mixed with coke. The gas emissions were measured in a different experiment with 1 g of pelletized samples heated isothermally in a horizontal tube furnace. With these experiments they concluded that the prereduction of manganese ores takes place by gaseous reduction mechanism and Boudouard reaction as the rate controlling step. Turkova et al.[17] studied correlation between CO-reactivity and porosity for three different manganese ores, CVRD, Assmang (Nchwaning) and Gabonese (Comilog). They used 200 ± 30 g samples heated in a vertical tube furnace with a heating rate of 10 °C/min. They used a gas composition of 70/30 CO/CO2 with a flow rate of 4 L/min. The ores were heated to three different max temperatures of 400 °C, 800 °C and 1100 °C. They found that ores with higher initial porosity will have a higher CO-reactivity, and that for Assmang (Nchwaning), a low porosity ore, an agglomerate with higher porosity will give a higher CO-reactivity. In addition, porosity increase below 400 °C was reported to be caused by the removal of bound water and in the temperature range 400 °C to 800 °C, the porosity increase was an effect of the prereduction of higher manganese oxides, swelling of the particle due to heating, and decomposition of carbonates. In another study, De Jesus and Tangstad[18] investigated the CO reactivity for lump manganese ore and briquettes at 1100 °C. A macro TGA was used with a gas composition of 70/30 CO/CO2 and heating rate of 10 °C/min. The lumpy ore was in the size range of 9 to 15 mm, while the briquettes were made of − 250 µm fines mixed with either molasses or clay. They concluded porosity was the biggest factor in CO reactivity, with molasses in briquettes decomposing at lower temperatures causing pores to form which resulted in the highest reactivity. Recently, extensive work on the reduction of Comilog and Nchwaning ores in CO/CO2 atmospheres and the analysis of the reaction rate of these ores has been carried out by Larssen et al.[11,19] They obtained thermogravimetric data for the reduction of Comilog and Nchwaning ores in CO/CO2 atmosphere were temperature, particle size and CO-concentration were investigated variables. It was reported that an increasing partial pressure of CO in CO/CO2 atmosphere promoted the reaction rate with an order of 0.7 and 1.5 for Comilog and Nchwaning ore, respectively. Activation energies of 17 kJ/mol and 63 kJ/mol were estimated for Comilog and Nchwaning ore, respectively. Carbonates in Nchwaning were found to decompose at temperature range 800 °C to 1000 °C and in addition, decreasing particle size promoted reaction rate for both ores.
Since the prereduction reactions are highly decisive for the total carbon, and energy consumption of the ferromanganese process, understanding the dependency of prereduction behavior on ore characteristics and furnace conditions is of paramount importance. This paper elucidates on the prereduction behavior of manganese ores with focus on Comilog an oxidized ore, Nchwaning a semi-oxidized ore and UMK a carbonate ore.
Experimental
Laboratory scale experiments were performed to investigate the prereduction behavior of three different ores. Two different types of experiments were performed, namely induction furnace simulating an industrial SAF and small scale thermogravimetric experiments.
Characterization of Raw Materials and Prereduced Products
The manganese ores investigated were UMK, Nchwaning and Comilog ores. Comilog exhibit high level of microporosity and is mainly composed of pyrolusite (β-MnO2), nsutite ((Mn4+)(1−x)(Mn2+)O(2−2x)(OH)2x (x = 0.06 to 0.07)) and cryptomelane (K(Mn4+7,Mn3+)O16) minerals. UMK and Nchwaning contains both braunite-I ((Mn2+,Mn3+)6(SiO4)O8) and braunite-II (Ca(Mn3+,Fe3+)6(SiO4)O8) minerals, Nchwaning contains more bixbyite ((Mn3+,Fe3+)2O3) and hematite (Fe2O3) and less carbonate minerals compared to UMK ore.[12] Characterization of ores and products of prereduction was for porosity and chemical compositions. Techniques used for characterization include pycnometry, X-ray fluorescence (XRF), permanganometric titration, thermogravimetric and combustion-IR methods. Elemental analysis (Mn, Fe, Si, Al, Ca, Mg, P, S, Ti, K and Ba) was measured with X-Ray fluorescence (XRF) fused bead technique by employing a Bruker AXS S4 Pioneer X-Ray fluorescence spectrometer (Billerica, US). Mn and Fe were given as total contents of the ores and other elements were converted into concentrations of their corresponding oxides, i.e., SiO2, Al2O3, CaO, MgO, TiO2, K2O and BaO in accordance with the stable oxides’ forms with a specific oxidation degree. The oxygen level (O/Mn ratio) of manganese ores and prereduced products was determined by permanganometric titration (ASTM 465-11:2017), in which excess oxygen above MnO is expressed as MnO2. The analysis of carbon concentration was done with a LECO (Combustion-IR) instrument, and the measured concentration of carbon was converted to CO2 concentration according to the stoichiometry, assuming that all carbon in the ore is in the form of CO2. Loss on ignition (LOI) was measured to a constant weight at 950 °C in air, thermogravimetrically. It is assumed that during the analysis, all carbonates are fully decomposed, water is evaporated, while the stable forms of manganese and iron are Mn3O4 and Fe2O3, respectively. The porosity of the solids ((1-(Apparent density/Absolute density)) was determined from the measurement of the absolute density (particle density) with Accupyc 1330 helium pycnometer and the measurement of the bulk density (apparent density) using a GeoPyc 1360 pcynometer. Prior to chemical analysis and experimental runs, the ores were dried at 100 °C for 24 hour to remove free water. Table I shows the chemical composition of the raw materials.
Induction Furnace Experiments
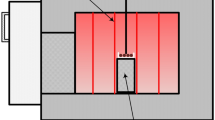
The extent of prereduction of the ores was investigated by simulating an industrial HCFeMn furnace in a 75-kVA open induction furnace. A temperature gradient develops during induction coil heating, with the highest temperature at the bottom of the furnace and decreasing temperature towards the furnace top. The raw materials in each experiment were stratified as layers into the graphite crucible of 150 mm outer diameter, 115 mm inner diameter, 400 mm outer height and approximately 380 mm inner depth and two setups were used. The first setup consisted of a 100 mm coke layer in the bottom of the crucible working as the coke bed in industrial furnace, a 200 mm ore layer placed on top of the coke bed and lastly, a 50 mm coke layer on the top of the ore working as insulation and inhibits charge re-oxidation. In the second setup, the 200 mm ore layer was replaced by a mixture of 90 wt pct ore + 10 wt pct coke reductant. A sketch of the induction furnace and graphite crucible containing the raw materials in the first setup and temperature gradients at coke bed temperatures of 1200 °C, 1300 °C and 1400 °C are as shown in Figures 1(a) and (b).
Schematic of the furnace set up (a) and temperature gradient (b). “(a) reprinted with permission from Ref. [23]”
The furnace crucible was heated at 25 °C/min from 25 to target temperatures of 1200 °C, 1300 °C and 1400 °C on top of the coke bed. During heating, the temperature is held for 30 minutes at 1200 °C to ensure sufficient prereduction prior to coke bed reduction reactions at higher temperatures. Final target temperatures were held constant for 60 minutes on top of the coke bed. Two type C tungsten/rhenium thermocouples encapsulated by alumina and graphite insulation tubes as shown in Figure 1(a) were used to measure the control and variable temperatures. The control thermocouple was located at the top of the coke bed and gave the reference temperature measurement. Likewise, the variable thermocouple was initially positioned on top of the coke bed and was used to measure temperatures at 20 mm interval distances away from the coke bed up to the top of the charge whilst maintaining a constant coke bed temperature. A constant temperature at each 20 mm interval position within the charge material was established by holding the variable TC for 2 minutes. Figure 1(b) show a typical temperature gradient at different coke bed temperatures. At the end of each experiment the furnace was shut down and the crucible was cooled for 24 hours. After cooling the crucible, samples were excavated manually from the same levels where the temperatures were measured, while the material in-between the temperature measurement points were discarded. A level then corresponds to the particles excavated at a given height inside the crucible. The collected samples were taken for chemical analysis and porosity measurements.
TGA Experiments
The prereduction of the UMK, Nchwaning and Comilog ores was investigated quantitatively by the thermogravimetric (TGA) technique. Total weight loss of the ore during prereduction was measured as a function of heating rate. The use of a TGA was meant to simulate the prereduction zone in an industrial ferromanganese furnace, with a constant supply of reducing gas CO/CO2. Ore particles in the size range 10 to 12.5 mm were subjected to different heating rates and a peak temperature of 1000 °C in a 70/30 CO/CO2 atmosphere. In the industrial furnace, most of the heat is developed around the electrode thereby giving a higher heating rate for particles close to the electrode, and lower heating rate for particles close to the furnace lining. To study the effect of heating rate in the furnace it was decided to use three different heating rates chosen as, 3 °C/min, closer to the furnace lining; 6 °C/min, between electrode and furnace lining; and 9 °C/min, close to the electrode.
An Entech VTF 80/15 vertical resistance tube furnace was used for the solid–gas reactions study. Ores occupying 80 mm height of the crucible were placed in a gas-tight stainless-steel double-wall crucible (L/Ø = 450/58 mm). The crucible was suspended from a scale (Mettler Toledo PR2003DR, Switzerland, 10 mg), with flexible gas inlet and outlet hoses connected at the top. The crucible was freely suspended, while the position of the furnace was adjustable vertically along a rail. The crucible was inside the furnace hot zone during the heating, while at the cooling stage the furnace was moved down. Furnace heating was regulated by an Eurotherm PID controller measured with a calibrated S-type thermocouple (Furnace Temperature), while charge temperature was measured with a K-type thermocouple (Crucible Temperature) sheathed in an alumina tube positioned in the center of the charge. The gases used in the experiments had a purity for CO, CO2 and Ar, of 99.97, 99.9992 and 99.999 vol pct, respectively. The gas flow in all experiments was regulated at 4 L/min 70/30 CO/CO2 by mass flow controllers (Bronkhorst High-Tech El-flow, Netherlands). The furnace was programmed to heat the sample from ambient temperature until 1000 °C at the given heating rates and the charge was then cooled under Ar flow to avoid reoxidation of the prereduced samples. In addition, the weight of the solid charge was measured with an external scale Ohaus Pioneer PA4202 (US) before and after the prereduction to confirm the net weight loss acquired from the TGA data. The sensitivity of the external scale was ± 0.01 g. After the experiments, prereduced samples were sieved through a sieving stack consisting of 10, 6.7, 4.75, 3.35, 1.6 and 0.5 mm sieves to determine ore decrepitation. From the sieved materials, prereduced ore samples were taken for chemical analysis. The decomposition of carbonates was investigated using the same experimental procedure at heating rate of 9 °C/min with experiments ending at 700, 800 and 900 °C for UMK ore and 900 °C for Nchwaning.
Oxides of manganese and iron display a wide range of solid solutions. Iron oxide (FeO) and co-existing MnO produced in prereduction has been observed by several researchers to dissolve in each other at high temperatures, forming FeO–MnO solutions.[11,21,24] Larssen et al.[11] studied the reduction of manganese ores in 80/20 CO/CO2 atmospheres utilizing a thermogravimetric furnace up to 1000 °C. The morphological observations in these studies[11,21] were in agreement with reduction of iron oxides ending with wüstite (FeO), which is stabilized by manganese in the monoxide phase. As such, the theoretical net weight loss of the ores during prereduction was estimated based on the chemical composition of the ore and corresponding equilibrium phases i.e., MnO and FeO at 1000 °C in the CO-containing gas atmosphere. The chemical composition of the ores in Table I, shows that Comilog has a high oxidation level (O/Mn ratio) equal to 1.93 (≈ MnO2), whereas Nchwaning and UMK are semi oxidized ores (predominantly Mn2O3) with oxygen level to 1.47 and 1.45, respectively. Similar oxygen levels have been reported in previous studies.[10,19,20] Fe concentration in manganese ores as measured by XRF was converted to Fe2O3, assuming that it is present in the ore as Fe(III). On complete prereduction, Fe concentration was converted to FeO, assuming that it is present there as Fe(II). The theoretical weight loss was calculated using LOI, O/Mn ratio, and Fe content of the ores, while accounting for the decomposition of carbonates as shown by Eq. [9]. The water content was calculated by subtracting the CO2 content given in the chemical analysis and calculated weight loss change from MnOx to Mn2O3 from the measured LOI. Table II shows the calculated theoretical weight loss for the ores.
The experimental data acquired from the macro thermogravimetric setup are reported in the form of weight change curves (wt pct) and its first derivative, the reaction rate (wt pct/s) as a function of temperature and time. To analyze the contribution to the overall weight loss of prereduction reactions, decomposition of carbonates and water evaporation, Fityk[25] was used to decompose the rate curves into these constituents. Fityk is a general purpose peak fitting program, which was used to analyze isothermal TGA data where reaction models are fitted to the experimental data and the model giving the best fit is assumed to be representative. The rate curves were automatically described using Gaussian curves, with the only limitation of no negative curves. The curves were checked by integrating the assumed contribution and verifying it against expected weight loss presented in Table II. The weight loss based on gas measurements only considers weight loss from reaction changing the CO/CO2 ratio, meaning only decomposition and prereduction. One prereduction reaction removes one mole of oxygen while producing one mole of CO2, whereas decomposition reaction removes one mole of CO2. Therefore, considering the molecular weights, the CO2 from decomposition reactions weighs 2.75 times more than oxygen removal from prereduction reactions. As such, for the rate curves analysis, the carbonate decomposition contribution was multiplied by the CO2 factor of 2.75.
Results and Discussions
Prereduction Behavior in Induction Furnace
Extent of prereduction of manganese ores
Experiments were conducted in a setup that simulates the industrial furnace process with temperatures on top of the coke bed held at 1200 °C, 1300 °C and 1400 °C and ores were unmixed and mixed with carbon on parallel studies. Figure 2 shows the extent of prereduction of Comilog, Nchwaning and UMK ores measured as the variation of O/Mn ratio as a function of temperature from samples excavated from the induction furnace. For ores not mixed with coke, UMK prereduces completely i.e., reaches O/Mn ration equal to 1.0 at temperatures lower than both Nchwaning and Comilog. Nchwaning and Comilog reaches an O/Mn ratio of 1 at around 950 °C, and UMK at 100 °C lower temperature. As such, UMK shows the highest extent of prereduction of the three ores. The variation of O/Mn ratio for excavations from experiments of ores mixed with coke show an increased extent of prereduction for all ores.
The prereduction of ores is affected by CO concentration in the reducing gas atmosphere. Several studies[10,11,19,26,27] have reported an increased reduction rate of manganese ores with increasing CO concentration in CO–CO2 atmosphere. The concentration of CO in the reducing gas atmosphere is generated from the metal producing reaction (reaction [8]) and/or the oxidation of C by CO2. In the induction furnace set up, no significant metal producing reaction was expected for Comilog, Nchwaning and UMK since the highest temperature peak of 1400 °C is below smelting temperatures of these ores.[28,29,30] Hence, less CO is produced from the metal producing reaction compared to pilot or industrial furnaces. In comparison to Nchwaning, the higher extent of prereduction in UMK is mainly because UMK contains a higher CO2 content as shown in Table I, and will release more CO2 during heating which oxidizes C thereby producing CO for prereduction of manganese oxides. The reason for the poor extent of prereduction for Comilog is mainly due to of a lack of CO. Since Comilog is not a carbonate ore, the lack of CO is due to the less CO2 produced that can react according to the Boudouard reaction and produce CO. The increased extent of prereduction when ores are mixed with solid carbon is due to increased oxidation of C by CO2 in the presence of coke, thereby increasing the rate of reduction by CO. Gasification of carbon in coke has been previously reported to be catalyzed by Mn3O4 and determines the overall rate of reduction.[15]
Porosity development and decomposition of carbonates
The porosity results shown in Figure 3 are for excavated ores, which were unmixed and mixed with coke and their corresponding residual carbonate content (given as CO2 content) as a function of temperature. The trendlines were calculated using a Lowess filter with a smoothing parameter of 0.35. It can be mentioned that the porosity is measured for single particles, and hence it is expected a large variation as the properties from particle to particle may be quite large.
Porosity development during heating and reduction shows that ore particles become more porous with increase in temperature as shown in Figure 3. This is in agreement with previous findings.[17,31] The peak porosity of Comilog and Nchwaning during reduction is around 800 °C to 1000 °C, whereas for UMK it is at 800 °C to 900 °C. Upon reaching a peak porosity, a downward trend for the porosity measurements with further increase in temperature is observed for UMK ore. This decrease in porosity is assumed to be due to the observed sintering of the particles. The presence of coke is expected to increase the porosity at lower temperatures, because of better prereduction, but the effect is unnoticeable considering the heterogeneity of the ores.
The carbonates present in the ores decompose endothermically. Several studies[10,32,33] have shown that the decomposition of carbonates is highly dependent on temperature and CO2 content in the reducing atmosphere, with increasing temperature and lowering the partial pressure of CO2 resulting in a greater extent of decomposition. The remaining CO2 content in the samples analyzed as a function of temperature is shown in Figure 3. It can be seen in seen that almost no CO2 content was observed from Comilog ore (Figure 3(a)), since the ore does not contain carbonate minerals and the analyzed CO2 could be from coke dust contaminating the ore particles. UMK ore (Figure 3(c)), shows a clear trend of decrease in CO2 content due to carbonate decomposition with increase in temperature up to 900 °C, beyond which carbonate decomposition is complete. This trend is also observed in Nchwaning ore, with some outliers since the initial content in Nchwaning is around 4.45 wt pct. Minerals distribution in Nchwaning ore is heterogeneous and the ore is in general characterized by very low porosity.[22,34] Therefore, the high carbonate content in some particles at elevated temperatures, as seen in Figure 3(b) could be pockets of undecomposed carbonates resulting from this heterogeneity. The rapid increase in porosity for UMK is believed to be due to decomposition of carbonates leaving voids in the ore particles.
Thermogravimetric Experiments
Prereduction behavior in 70/30 CO/CO2 atmosphere
The experimental weight loss data acquired from the TGA setup are reported and utilized in calculation of change in O/Mn ratio and its first derivative as reaction rate (wt pct/s) all as functions of temperature. The variation of weight loss with temperature for non-isothermal reduction of Comilog, Nchwaning and UMK is shown in Figure 4(a). The corresponding O/Mn ratio calculated through the mass loss as a function of temperature is shown in Figure 4(b). The mass loss follows the increase in sample temperature. Comilog ore which has a higher initial O/Mn ratio of 1.93 forms a “Z-shape” in the weight loss vs temperature curve. This is due to exothermic reduction reactions, which cause the temperature to increase above the programmed temperature and is characteristic of MnO2–ores.[17,19] Some deviation is observed in Figure 4(a), where the “Z-shape” curve for Comilog ore at 6 °C/min reaches a higher temperature than that at 9 °C/min. At 9 °C/min the exothermic reactions initiate at lower temperatures compared to 6 °C/min, thus more MnO2 has been consumed and this results in a lower exothermic peak temperature. Nchwaning and UMK are Mn2O3 based ores and their prereduction starts from an initial O/Mn ratio of 1.47 and 1.45, respectively. Figure 4(a) shows that UMK has the highest weight loss followed by Comilog and lastly, Nchwaning. This trend is in accordance with theoretical weight loss shown in Table II. A greater part of the weight loss from UMK is from carbonate decomposition, which is approximately 4 times the weight loss contribution from prereduction as shown in Table II, whereas in Comilog its mainly from prereduction reactions and evaporation as it contains higher manganese oxides MnO2 and around 3.8 wt pct water content. Nchwaning is an Mn2O3 ore, with lower carbonate content compared to UMK has weight loss contribution from prereduction and carbonate decomposition.
The very exothermic reactions observed when prereducing Comilog ore occurs very close to 500 °C at heating rates 6 °C/min and 9 °C/min as seen in Figure 4. This exothermic peak rapidly increases the temperature close to or exceeding 800 °C. At a heating rate of 3 °C, the exothermic peak is absent as the furnace is able to tune the temperature with less exothermic reactions per minute. Weight loss in Comilog ore starts at approximately 200 °C and Comilog ore has an O/Mn ratio very close to 1 at 800 °C. Between 600 °C and 800 °C, one can see that the lower heating rate gives a lower O/Mn ratio at a given temperature. Nchwaning ore shows a clear distinction of the effect of heating rate on prereduction, with a lower heating rate of 3 °C/min giving rise to a higher extent of prereduction. The prereduction of Nchwaning ore commences at about 350 °C and the prereduction is complete by 1000 °C. On the other hand, the prereduction of UMK ore commences at slightly higher temperature at 500 °C compared to Nchwaning ore and is completely prereduced by 900 °C. The corresponding reaction rate (wt pct/s) curves as a function of temperature for all the three heating rate scenarios in the studied ores is shown in Figure 5. In general, the reaction rate is highly influenced by heating rate, with decreasing heating rate resulting in longer time at each temperature. The rate curves for Comilog ore in Figure 5(a) shows that at 6 °C/min, the exothermic peak reaches a higher temperature compared to 9 °C/min heating rate. The rate curves in Figure 5(b) for Nchwaning ore are characterized by the highest peak occurring at about 900 °C. The lower peaks at temperatures less than 900 °C become less pronounced with decreasing rate. As shown in Figure 5(c), the prereduction of UMK ore occurred with two major distinctive steps, the first one mostly occurring at about 700 °C, and the second one at the higher temperature 900 °C. It is also observed that the peaks in UMK reduce in size with decreasing heating rate, due to the longer time at each temperature.
An interesting phenomenon that could affect the TGA weight loss data and subsequently the rate curves, is carbon deposition. This may be difficult to detect from weight behavior when prereduction reactions occur simultaneously. According to thermodynamics, the Boudouard (CO2 + C = 2CO) reaction is shifted to the left below 700 °C resulting in carbon deposition, whereas at higher temperatures it will proceed to the right. A high CO/CO2 ratio enhances carbon deposition. Larssen[22] conducted experiments with quartz in 80/20 CO/CO2 atmospheres and found that carbon deposition was initiated at temperatures close to 400 °C and continued with increase in temperatures. At temperatures above 780 °C gasification of the deposited carbon was observed. As such, it is believed that the initial peaks observed in the reaction rate curves for mostly Nchwaning ore and UMK ore, which increase with increasing heating rate, as shown in Figures 5(b) and (c) are due to carbonate decomposition. Prereduction reactions in Comilog ore start at relatively low temperatures as shown by the weight loss curve in Figure 4(a). This causes CO to be consumed at low temperatures, thus the driving force for the carbon deposition is lower in this case. Therefore, it is believed that prereduction of Comilog ore is not affected by carbon deposition. No carbon was observed in the reduced samples in all experiments; hence any deposited carbon had been gasified during the experiments. However, gasification of carbon is expected to occur simultaneously as the higher reduction rates in these experiments and would be obscured by the reduction reactions.
Evaluation of the reactions occurring at the distinctive peaks in Nchwaning and UMK was done by conducting non-isothermal experiments at 9 °C/min in 70/30 CO/CO2 gas atmosphere, with final temperatures at the peak regions. The final temperatures were 700, 800 and 900 °C for UMK whereas for Nchwaning the experiment was ended at 900 °C. The chemical composition of samples from these experiments in addition to the initial experiments are shown in Table III. The chemical composition shows significant decrease in carbonate content for Nchwaning and UMK at the investigated peak temperatures. Therefore, the percentage carbonate decomposed from Nchwaning and UMK was calculated from initial values and the results are shown in Figure 6. These results show that the described distinctive peaks in UMK and Nchwaning are mainly due to decomposition of carbonates in addition to prereduction reactions.
Analysis of the constituents of TGA rate curves
The change of mass loss during TGA experiments corresponds to removal of oxygen from higher manganese oxides and iron oxides i.e., prereduction reactions, decomposition of carbonates and water evaporation. As such, there are several reactions involved and it is not straightforward to isolate effects of these individual reaction components. Therefore, to establish the contributions of these components, Fityk[25] a general purpose peak or model fitting software was used to decompose the rate curves.
The constituents contributing to the overall rate of weight loss in Comilog ore are water evaporation and prereduction reactions shown in Figure 7, at different heating rates. Temperature profiles are indicated on the secondary axis as a function of time. When looking at the decomposed rate curves for 3 °C/min, the water evaporation happens virtually at the same time as the main reduction rate peak. Considering all the heating rates, integration of the water loss curves gives total weight of 3.24, 3.88 and 3.84 wt pct at heating rates of 3, 6, 9 °C/min, respectively. These values show a high accuracy in relation to 3.8 wt pct water content in the ore as presented in Table II. The temperature range for evaporation is 200 to 300 °C, which is in agreement with previously reported evaporation temperature range.[22] At 6 and 9 °C/min, the reduction reaction can be seen starting at low temperatures and the crucible temperature increases in accordance with exothermic reactions, but it is not until the water is completely evaporated that the main bulk of the reaction occurs, giving a very exothermic peak.
The constituents contributing to the overall rate of weight loss in Nchwaning ore are decomposition of carbonates and prereduction reactions shown in Figure 8, at different heating rates. Temperature profile indicated on the secondary axis as a function of time show an endothermic dip in the sample temperature at 900 °C, which corresponds to where the carbonate contribution peaks are. The expected weight loss from decomposition of carbonates is 4.45 wt pct as presented in Table II. Integration of the rate curves of the carbonate contribution gives total weight loss of 4.21, 3.91 and 3.47 wt pct at heating rates of 3, 6, 9 °C/min, respectively. Therefore, the weight loss due to carbonate contribution decreases with increasing heating rates. The majority of the reduction, and exothermic reactions, occur at temperatures below 800 °C as shown by reduction constituents.
Figure 9 shows the contributions of decomposition of carbonates and prereduction reactions to the overall rate of weight loss in UMK ore and their changes with heating rate. Temperature profiles for UMK indicated on the secondary axis as a function of time show two endothermic dips in the sample temperature at 700 °C and 900 °C. The height of the first peak at 700 °C is shown to decrease with increasing heating rate and is attributed to both prereduction and decomposition of carbonates. As previously shown in Figure 6, about 20 wt pct carbonates are decomposed at this first peak, and the O/Mn ratio is 1.29 as presented in Table III. Therefore, in the first peak reduction reactions happen in parallel with the carbonate decomposition reaction. On the other hand, the height of the second peak at 900 °C appears to be unaffected by the heating rate. At this peak, a greater proportion of carbonates is decomposed as shown in Figure 6 and the prereduction reactions are complete as O/Mn ratio is 1.0 as shown in Table III. The curves in Figure 9 reveal that UMK is fully reduced by 900 °C and the carbonates decompose in two steps and this is supported by the chemical analysis in Table III and variation of the decomposition of carbonates with temperature shown in Figure 6. Based on the chemical analysis of UMK ore in Table I, the carbonates are assumed to be dolomitic and previous studies[32,33] have shown dolomite to decompose in two distinct steps with temperature zones depending on the type of mineral existing around it. Integration of the rate curves of the carbonate contribution gives total weight loss of 14.7, 14.0 and 13.8 wt pct at heating rates of 3, 6, 9 °C/min, respectively. As seen from Table II reduction of iron and manganese oxides in UMK ore is expected to contribute about 5 wt pct and decomposition of carbonates about 16 wt pct to the overall weight loss. The curves presented have been verified to match the total weight loss expected from their constituents and the slight variations could be attributed to heterogeneity of ores and uncertainty in chemical analysis. In conclusion, the two endothermic peaks observed in the rate of weight loss curves in UMK are essentially due to decarbonation of dolomite and reduction reactions for the first peak and mainly decarbonization of calcite in the second peak.
Decrepitation of ores
Figure 10 shows the particle size distribution for Comilog, Nchwaning and UMK ores heated to 1000 °C in 70/30 CO/CO2.
The Nchwaning ore particle size distribution curve has a similar shape as the UMK ore compared to the Comilog ore curve when heated in CO/CO2. However, in the finer size fractions below 4.75 mm Nchwaning ore generates more fines compared to UMK ore. The distribution below 10 mm is shallow for Nchwaning ore, meaning that the particles are distributed more equally between the finer mesh sizes. This signifies that when Nchwaning ore decrepitates it produces more fines than medium sized particles whereas when UMK ore decrepitates it tends to crack into larger particles, as opposed to generating fines. There is no apparent correlation between heating rate and decrepitation for Nchwaning ore. The effect of heating rate on decrepitation on UMK ore shows that an increase in heating rate results in increased decrepitation. On the contrary, Comilog ore shows that increase in heating rate results in decreased decrepitation. Though there is a difference on the effect of heating rate on decrepitation when comparing UMK ore and Comilog ore, the significance of heating rate on decrepitation of UMK ore is not high. From Figure 10, the fraction of particles with size range lower than 10 mm are 75 to 90 pct, 35 to 42 pct and 48 to 60 pct for Comilog, Nchwaning and UMK ores, respectively. There is a significant difference in size distribution for all the three ores in CO/CO2, which shows that Comilog ore produces the most fines followed by Nchwaning ore and lastly UMK ore. Higher decrepitation of Comilog in comparison to other ores is in agreement with previous investigations.[22,31] Comilog ore has been reported to have lower mechanical strength and high initial porosity in the range 30 to 50 pct and Nchwaning ore is characterized by a higher mechanical strength and very low porosity (0 to 1 pct).[34] As such Comilog is expected to decrepitate the most. Larssen[22] measured the decrepitation of Comilog and Nchwaning ores, and showed the same correlation between lower heating rates and an increase in decrepitation for Comilog ore. In another study, Biørnstad[31] studied the decrepitation for UMK, Nchwaning and Comilog and showed that Nchwaning decrepitates more than UMK, which is contrary to findings in this work, however they are both in the same area.
Conclusions
From the obtained results it can be conclude that:
-
(1)
The decomposition of carbonates lead to better prereduction in the induction furnace experiments, due to the CO produced in the Boudouard reaction. This is the reason why UMK ore has a better prereduction compared to Nchwaning ores which is low in carbonates and Comilog ore which is known to have a higher CO reactivity at similar CO contents. Prereduction of ores mixed with solid carbon resulted in improved prereduction compared to ore only in induction furnace experiments.
-
(2)
Analysis of the rate curves from TGA shows that decomposition of carbonates in UMK ore is a two-step process with peak temperatures at 700 °C and 900 °C whereas for Nchwaning ore, it is a single step with peak temperature at 900 °C. The first endothermic peak in UMK ore is a combination of decomposition of carbonates and prereduction reactions whereas the second peak is mainly carbonate decomposition and O/Mn ratio is 1.0 showing that prereduction of higher manganese oxides is complete prior to second decomposition reaction. For Nchwaning, the endothermic peak is mainly decomposition of carbonates and prereduction reaction is almost complete with an O/Mn ratio of 1.08.
-
(3)
Comilog ore produces most fines followed by Nchwaning and lastly UMK on decrepitation.
Change history
31 October 2022
Missing Open Access funding information has been added in the Funding Note.
References
S.E. Olsen, M. Tangstad, and T. Lindstad: Production of Manganese Ferroalloys, Tapir Academic Press, Trondheim, 2007.
K.N. Swamy, D.G.C. Robertson, P. Calvert, and D. Kozak: in The 9th International Ferroalloys Congress. Quebec City, Canada. 2001, pp. 293–301.
A. Ahmed, H. Haifa, M.K. El-Fawakhry, H. El-Faramawy, and M. Eissa: J. Iron Steel Res. Int., 2014, vol. 21, pp. 666–72.
T. Mukono, J.E. Gjøvik, H. Gærtner, M. Wallin, E. Ringdalen, and M. Tangstad: in 16th International Ferroalloys Congress. Trondheim. 2021. pp. 1–14.
O.I. Ostrovski and T.J.M. Webb: ISIJ Int., 1995, vol. 35, pp. 1331–39.
A. Roine: HSC Chemistry Software, v 10.0.4.3, Metso Outotec, Pori, 2021.
R. Ishak, and M. Tangstad: in The 11th International Ferroalloys Congress. New Dehli, India. 2007. pp. 268–80.
B. Sorensen, S. Gaal, M. Tangstad, E. Ringdalen, R. Kononov, and O. Ostrovski: in 12th International Ferroalloys Congress. Helsinki, Finland. 2010. pp. 439–48.
L.A. Corathers: Minerals Yearbook: Manganese. United States Geological Survey. 2008.
T.L. Schanche and M. Tangstad: Minerals, 2021, https://doi.org/10.3390/min11101097.
T.A. Larssen, D. Senk, and M. Tangstad: Metall. Mater. Trans. B, 2021, vol. 52, pp. 363–81.
M.J. Peterson, J.R. Manuel, and S. Hapugoda: Appl. Earth Sci. Trans. Inst. Min. Metall., 2020, vol. 6, pp. 1–21.
J. Ostwald: Ore Geol. Rev., 1988, vol. 4, pp. 3–45.
J. Gutzmer and N.J. Beukes: Ore Geol. Rev., 1996, vol. 11, pp. 405–28.
G.J.W. Kor: Metall. Mater. Trans. B, 1978, vol. 10, pp. 1–5.
K.S. Abdel Halim, M. Bahgat, M.B. Morsi, and K. El-Barawy: Ironmak. Steelmak., 2011, vol. 38, pp. 279–84.
K. Turkova, D. Slizovskiy, and M. Tangstad: ISIJ Int., 2014, vol. 54, pp. 1204–08.
L.G.M. de Jesus and M. Tangstad: ISIJ Int., 2020, vol. 60, pp. 2129–33.
T.A. Larssen, D. Senk, and M. Tangstad: Metall. Mater. Trans. B, 2021, vol. 52, pp. 2087–2100.
D. Ngoy, D. Sukhomlinov, and M. Tangstad: ISIJ Int., 2020, vol. 60, pp. 2325–331.
M.S. Fahim, H. El Faramawy, A.M. Ahmed, S.N. Ghali, and A.E.H.T. Kandil: J. Miner. Mater. Charact. Eng., 2013, vol. 01, pp. 68–74.
T.A. Larsen: Prereduction of Comilog and Nchwaning ore. Ph.D. Thesis. Dep. Mater. Sci. Eng. NTNU. 2020.
T. Mukono, M. Wallin, and M. Tangstad: Metall. Mater. Trans. B, 2022, https://doi.org/10.1007/s11663-022-02441-5.
T.I. Schanche, and M. Tangstad: in 16th International Ferroalloys Congress. 2021. pp. 27–29.
M. Wojdyr: J. Appl. Crystallogr., 2010, vol. 43, pp. 1126–28.
N. Anacleto, O. Ostrovski, and S. Ganguly: ISIJ Int., 2004, vol. 44, pp. 1480–87.
Y.B. Gao, H.G. Kim, and H.Y. Sohn: Trans. Inst. Miner. Metall. Sect. C Miner. Process. Extr. Metall., 2012, vol. 121, pp. 109–16.
E. Ringdalen, M. Tangstad, and T. Brynjulfsen: in 14th International Ferroalloys Congress. Kiev, Ukraine. 2015. pp. 436–45.
S. Gaal, E. Ringdalen, D. Vaganov, and M. Tangstad: in 8th International Conference on Molten Slags, Fluxes and Salts. Santiago, Chile. 2009. pp. 101–9.
S. Gaal, D. Lou, S. Wasbø, B. Ravary, and M. Tangstad: in 11th International Ferroalloys Congress. 2007. pp. 247–57.
O. Biørnstad: Decrepitation of Comilog, Assmang and UMK manganese ores during prereduction. MSc Thesis. Dep. Mater. Sci. Eng. NTNU. 2020.
S. Gunasekaran and G. Anbalagan: Bull. Mater. Sci., 2007, vol. 30, pp. 339–44.
A.I. Rat’Ko, A.I. Ivanets, A.I. Kulak, E.A. Morozov, and I.O. Sakhar: Inorg. Mater., 2011, vol. 47, pp. 1372–77.
M. Tangstad, P. Calvert, H. Brun, and A.G. Lindseth: in The 10th International Ferroalloys Congress. Cape Town, South Africa. 2004. pp. 213–22.
Acknowledgments
This work is part of the PreMa project, which has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No 820561 and industry partners: Eramet, Ferrogroble, Transalloys, OFZ and Outotec.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mukono, T., Reiersen, H.S., Schanche, T.L. et al. Prereduction Behavior of Manganese Ores With Solid Carbon and in CO/CO2 Gas Atmosphere. Metall Mater Trans B 53, 3292–3305 (2022). https://doi.org/10.1007/s11663-022-02611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02611-5