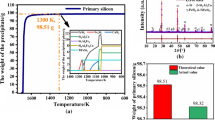
Abstract
Iron (Fe) in metallurgical-grade silicon (MG-Si) is the main metal impurity and is difficult to remove. Strengthening the segregation of impurity Fe at the solid–liquid interface is the key to purifying MG-Si; therefore, a refinement method for the directional solidification of hypereutectic silicon–titanium (Si–Ti) alloy was proposed, and the segregation ability was quantified by calculating the equilibrium segregation coefficient of impurity Fe. By analyzing the microstructure, segregation thermodynamics, and equilibrium segregation coefficient of impurity Fe in a Si-rich layer, it was confirmed that the segregation ability of impurity Fe during Si–Ti alloy refining was stronger than that of MG-Si. The results showed that impurity phases containing Fe precipitates in the Si-rich layer included τ5 and τ1 phases. After introducing Ti into the Si melt, the equilibrium segregation coefficient of Fe impurities was significantly reduced, and the equilibrium segregation coefficient of Si–10 wt pct Ti–0.396 wt pct Fe was k0Fe = 8.68 × 10−7, which was much lower than that of MG-Si (6.4 × 10−6). During the directional solidification and refining of Si–10 wt pct Ti–0.396 wt pct Fe, the removal rate of impurity Fe increased from 90.5 to 96.75 pct of MG-Si to 99.86 pct. This work helps provide understanding of the segregation behavior of impurities at the solid–liquid interface during the directional solidification of Si–Ti alloys and lays a theoretical foundation for the deep removal of impurities.









Similar content being viewed by others
References
L. Ma, Z. Yu, W. Ma, S. Qing, and J.J. Wu: Silicon Neth., 2019, vol. 11, pp. 1383–91. https://doi.org/10.1007/s12633-018-9937-6.
S. Yang, X. Wan, K. Wei, W. Ma, and Z. Wang: J. Clean. Prod., 2021, https://doi.org/10.1016/j.jclepro.2020.125525.
S. Han, N. Tan, K. Wei, and W. Ma: Sep. Purif. Technol., 2022, https://doi.org/10.1016/j.seppur.2021.119815.
F. Chigondo: Silicon Neth., 2018, vol. 10, pp. 789–98. https://doi.org/10.1007/s12633-016-9532-7.
A.F.B. Braga, S.P. Moreira, P.R. Zampieri, J.M.G. Bacchin, and P.R. Mei: Sol. Energy Mater. Sol. Cells, 2008, vol. 92, pp. 418–24. https://doi.org/10.1016/j.solmat.2007.10.003.
S. Yang, X. Wan, K. Wei, W. Ma, and Z. Wang: Waste Manag., 2021, vol. 120, pp. 820–27. https://doi.org/10.1016/j.wasman.2020.11.005.
S. Yang, X. Wan, K. Wei, W. Ma, and Z. Wang: Miner. Eng., 2021, https://doi.org/10.1016/j.mineng.2021.106966.
H. Chen, K. Morita, X. Ma, Z. Chen, and Y. Wang: Sol. Energy Mater. Sol. Cells, 2019, https://doi.org/10.1016/j.solmat.2019.110169.
S. Pizzini: Sol. Energy Mater. Sol. Cells, 2010, vol. 94, pp. 1528–33. https://doi.org/10.1016/j.solmat.2010.01.016.
J.M. Bablin, A.C. Crawford, D.C. DeMoulpied, and L.N. Lewis: Ind. Eng. Chem. Res., 2003, vol. 42, pp. 3555–65. https://doi.org/10.1021/ie020334s.
B. Gillot, G. Weber, H. Souha, and M. Zenkouar: J Alloy Compd., 1998, vol. 270, pp. 275–80. https://doi.org/10.1016/S0925-8388(98)00527-1.
Y. Li and L. Zhang: Sep. Purif. Rev., 2021, vol. 50, pp. 115–38. https://doi.org/10.1080/15422119.2019.1623253.
Y. Li, W. Chen, J. Lu, X. Lei, and L. Zhang: J. Electron. Mater., 2021, vol. 50, pp. 1386–96. https://doi.org/10.1007/s11664-020-08651-4.
A. Hoseinpur, S. Andersson, K. Tang, and J. Safarian: Langmuir, 2021, https://doi.org/10.1021/acs.langmuir.1c00876.
F. Xi, S. Li, W. Ma, Z. Chen, K. Wei, and J.J. Wu: Hydrometallurgy, 2021, vol. 201, p. 10553. https://doi.org/10.1016/j.hydromet.2021.105553.
G. Qian, L. Sun, H. Chen, Z. Wang, K. Wei, and W. Ma: J. Alloy Compd., 2020, https://doi.org/10.1016/j.jallcom.2019.153300.
K. Zhu, J. Hu, W. Ma, K. Wei, T. Lv, and Y. Dai: J. Cryst. Growth, 2019, vol. 522, pp. 78–85. https://doi.org/10.1016/j.jcrysgro.2019.05.012.
J. Hu, K. Zhu, K. Wei, W. Ma, and T. Lv: J. Alloy Compd., 2020, https://doi.org/10.1016/j.jallcom.2019.152621.
L. Zhou, K. Zhu, T. Yan, J. Hu, K. Wei, and W. Ma: Metall. Mater. Trans. B, 2022, vol. 53B, pp. 1–12. https://doi.org/10.1007/s11663-021-02395-0.
P. Li, S. Ren, D. Jiang, J. Li, L. Zhang, and Y. Tan: J. Cryst. Growth, 2016, vol. 437, pp. 14–19. https://doi.org/10.1016/j.jcrysgro.2015.12.007.
Y. Li, B. Ban, J. Li, T. Zhang, X. Bai, J. Chen, and S. Dai: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 542–44. https://doi.org/10.1007/s11663-015-0291-4.
J. Li, B. Ban, Y. Li, X. Bai, T. Zhang, and J. Chen: Silicon Neth., 2017, vol. 9, pp. 77–83. https://doi.org/10.1007/s12633-014-9269-0.
F. Weitzer, J.C. Schuster, M. Naka, F. Stein, and M. Palm: Intermetallics, 2008, vol. 16, pp. 273–82. https://doi.org/10.1016/j.intermet.2007.10.006.
Q.C. Zou, J.C. Jie, J.L. Sun, T.M. Wang, Z.Q. Cao, and T.J. Li: Sep. Purif. Technol., 2015, vol. 142, pp. 101–07. https://doi.org/10.1016/j.seppur.2015.01.005.
J. Yang, J. Zhang, Y. Dai, J. Ma, F. Li, F. Bian, J. Mi, and B. Sun: Comput. Mater. Sci., 2015, vol. 109, pp. 1–48. https://doi.org/10.1016/j.commatsci.2015.05.033.
M. Zhu, S.Y. Yue, K. Tang, and J. Safarian: ACS Sustain. Chem. Eng., 2020, vol. 8, pp. 15953–15966. https://doi.org/10.1021/acssuschemeng.0c05564.
W. Yu, Y. Xue, J. Mei, X. Zhou, M. Xiong, and S. Zhang: J. Alloy Compd., 2019, vol. 805, pp. 198–204. https://doi.org/10.1016/j.jallcom.2019.07.089.
T. Yoshikawa, K. Morita, S. Kawanishi, and T. Tanaka: J. Alloy Compd., 2010, vol. 490, pp. 31–41. https://doi.org/10.1016/j.jallcom.2009.09.190.
X. Deng, S. Li, J. Wen, K. Wei, M. Zhang, X. Yang, and W. Ma: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 625–32. https://doi.org/10.1007/s11663-020-02028-y.
D.P. Tao: Thermochim. Acta, 2000, vol. 363, pp. 105–13. https://doi.org/10.1016/S0040-6031(00)00603-1.
F. Yang, J. Wu, and W. Ma: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 2381–90. https://doi.org/10.1007/s11663-020-01895-9.
D.P. Tao: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 2232–46. https://doi.org/10.1007/s11663-014-0154-4.
Y. Li, Q.F. Gu, Q. Luo, Y. Peng, S.L. Chen, K.C. Chou, X.L. Wang, and Q. Li: Mater. Des., 2016, vol. 102, pp. 78–90. https://doi.org/10.1016/j.matdes.2016.03.144.
Y. Zhang, Y. Lei, W. Ma, H. Wang, Y. Hu, K. Wei, and S. Li: J. Alloy Compd., 2020, https://doi.org/10.1016/j.jallcom.2020.153989.
F. Huang, R. Chen, J. Guo, H. Ding, and Y. Su: Sep. Purif. Technol., 2017, vol. 188, pp. 67–72. https://doi.org/10.1016/j.seppur.2017.06.073.
D.H. Liu, X.D. Ma, Y.Y. Du, T.J. Li, and G.L. Zhang: Mater. Res. Innov., 2010, vol. 14, pp. 361–64. https://doi.org/10.1179/143307510X12820854748674.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant Nos. U1902219 and U1702251), Special Funds of State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization (Grant No. CNMRCUTS1604), Reserve Talents of Young and Middle-Aged Academic and Technical Leaders in Yunnan Province (Grant No. 2018HB009), Yunnan Outstanding Youth Science Foundation (Grant No. 202101AV070007), Sichuan Science and Technology Program (Grant No. 2021YJ0548), and Research Project of Panzhihua University (Grant No. 2020ZD002).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Zhu, K., Deng, X. et al. Segregation Behavior of Impurity Iron in Primary Silicon During Directional Solidification of a Hypereutectic Silicon–Titanium Alloy. Metall Mater Trans B 53, 2262–2271 (2022). https://doi.org/10.1007/s11663-022-02527-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02527-0