Abstract
The effects of basicity and Al2O3 content on the chemistry of phases in iron ore sinter containing ZnO were investigated by Rietveld analysis of the X-ray diffraction (XRD) patterns. Bulk composition analysis was carried out using inductively coupled plasma-atomic emission spectroscopy (ICP-AES) and wet-chemical analysis. The composition of each phase was investigated using a scanning electron microscope-energy dispersive X-ray analyzer (SEM-EDX). It was found that ZnO exists in the franklinite and the silicoferrite of calcium and aluminum (SFCA) phases. With increasing ZnO content, the phase fraction of the franklinite increased, while the fraction of SFCA slightly increased. When ZnO content was fixed at 1 wt pct and basicity increased, the fraction of franklinite decreased and that of SFCA increased. Here, the solubility of ZnO in the SFCA increased. As the Al2O3 content increased, the fraction of franklinite decreased and that of SFCA increased, while ZnO content in the SFCA did not change significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recycling by-products generated from the integrated iron- and steelmaking industry has drawn attention from the vantage point of environmental and economic issues. Charging of iron-containing industrial by-products, such as scrap and dust in the blast furnace (BF), is useful to reduce CO2 emission from the ironmaking process as well as to increase economic competency.[1,2,3,4] For example, BF dust can be considered as a source of iron in BF operation, because it contains 15 to 40 wt pct of Fe. Recycling of BF dust has been generally applied to the sintering process.[5,6,7] BF dust is mostly composed of carbon, CaO, SiO2, and iron oxides, but it contains some minor constituents such as Al2O3, MgO, ZnO, and so on. Among them, many researchers pointed out that zinc in iron ore sinter may cause accumulation of ZnO in the iron ore sinter and wear of the refractory material during the BF operation.[8,9,10]
When iron ore sinter containing Zn is charged in the BF, or pulverized coal containing Zn is injected into the BF through tuyere nozzles, Zn vapor is generated in the relatively lower part of the BF. While traveling with the gas stream to the upper part of the BF, Zn vapor is oxidized and condensed in the charged burdens or the refractory materials. As this sequence is repeated, ZnO is accumulated in the iron ore sinter and the refractory materials.[8,9] As ZnO content increases in the refractory material, damage of the refractory material may occur.[10] If we consider the maximum use of various dust (not only BF dust but also basic oxygen furnace dust[11,12] and electric arc furnace dust[13,14]), it is important to know the chemistry of iron ore sinter containing ZnO.
On the other hand, phases in the iron ore sinter are complex and mosaic structure is frequently observed. For the quantitative phase analysis of the iron ore sinter, the image analysis technique on the cross-sectional image has been generally applied[15,16] and the chemical composition of each phase has been determined using a scanning electron microscope-energy dispersive X-ray analyzer (SEM-EDX). However, the cross-sectional image does not provide adequate volume fractions of the phases so that an alternative technique for quantitative phase analysis should be considered. Hamilton et al. suggested that Rietveld analysis of the X-ray diffraction (XRD) patterns of the bulk sample would be the most promising method for the quantitative phase analysis,[17] and several researchers have adopted this method for the quantitative phase analysis.[18,19,20,21,22,23,24] For example, Webster et al. investigated the phase transition at high temperatures using in-situ synchrotron XRD.[18,19,20,21,22,23] More recently, Takayama et al. quantitatively analyzed the phases in iron ore sinters from sinter pot tests.[24]
Generally, iron ore sinter is composed of hematite, magnetite, silicoferrite of calcium and aluminum (SFCA), dicalcium silicate (C2S), etc. Since Hancart et al.[25] reported that SFCA was the major bonding phase in the iron ore sinter, many researchers[26,27,28] have explored the composition range of SFCA. Inoue and Ikeda[26] suggested a substitution plane of SFCA; the substitution plane, called the “CCC plane,” was defined by the end members of CaO·3Fe2O3-CaO·3Al2O3-CaO·SiO2. Dawson et al.[27] reported the substitution plane as CaO·2Fe2O3-CaO·2Al2O3-CaO·3SiO2. Patrick and Pownceby[28] suggested the substitution plane as CaO·3Fe2O3-CaO·3Al2O3-4CaO·3SiO2. Especially, calcium-ferrite–based materials are sensitive to several factors such as the composition,[29,30,31,32,33,34,35,36] thermal history,[37] and gas atmosphere.[18,38,39] Therefore, it is important to control the factors and analyze the samples exactly.
In the iron ore sinter, Zn may form zinc ferrite or can be dissolved in other phases. However, no study has been reported yet on the phase equilibria and solubility of Zn in other phases of the iron ore sinter. In this study, the form of Zn in the iron ore sinter is identified by using the Rietveld analysis of the XRD patterns of the iron ore sinters and SEM-EDX analysis of the phases. With varying the bulk sample composition, the effects of basicity and Al2O3 content on the chemistry of the phases in the iron ore sinter are investigated.
Experimental
In the present study, mostly reagent grade chemicals (CaCO3, Fe2O3, SiO2, Al2O3, and ZnO, 99 pct purity, < 100 μm) were used. CaCO3 was calcinated to obtain CaO at 1273 K (1000 °C) for 2 hours. CaO was then crushed and particles of 53 to 100 μm were used. Commercial grade coke, which was provided by POSCO, was used as a carbonaceous material. Coke particles, which were crushed and sieved to the same size as CaO, were selected and used in the present experiments. The chemicals at a predetermined ratio were mixed and pelletized in a drum mixer at 250 rpm for 2 hours, while distilled water was sprayed by 7 wt pct. Then, iron ore green pellets of 2 to 4 mm were selected and used for the lab-scale sintering simulation experiments. Table I shows the nominal composition of the sample pellets, and Table II summarizes the chemical composition of the coke used in this study. After experiments, the chemical composition of the sample was analyzed using inductively coupled plasma-atomic emission spectroscopy (ICP-AES) and wet-chemical analysis.
Figure 1 shows a schematic illustration of the experimental apparatus. An electric resistance furnace of a maximum temperature of 1573 K (1300 °C) was used. A vertical alumina reaction tube (ID: 60 mm, OD: 70 mm, and length: 1000 mm) was placed in the furnace, and both sides of the tube were sealed with stainless cooling jackets. An alumina tube (ID: 47 mm, OD: 54 mm, and length: 314 mm) was attached to the bottom jacket with O-rings and another alumina tube (length: 100 mm) was placed above it. Between the two alumina tubes, a stainless-steel mesh was placed to support the sample particles. At the bottom, alumina balls of 2 mm in diameter were charged to the height of 20 mm to prevent the dropping of molten phases during experiments. Then, pellets were charged in a 60-mm-thick layer. Finally, alumina balls of 2 mm in diameter were added on the top in a 20-mm thickness. Twenty millimeters above the sample particles, a heat exchanger composed of alumina balls of 10 mm in diameter was placed to prevent the chilling of the sample particles by rapid gas flows. To monitor temperature variation during experiments, two type-R thermocouples were buried in the sample pellets: one (TC-1) at a position 20 mm below from the top surface of the pellet layer and the other (TC-2) 20 mm below from TC-1.
Once the sample assembly was installed, it was heated to 1273 K (1000 °C) at a heating rate of +10 K/min (+10 °C/min) while flowing N2 gas from the top of the reaction tube at a flowing rate of 300 mL/min STP. At 1273 K (1000 °C), 79 pct N2-21 pct O2 gas mixture was injected at a flowing rate of 20 L/min STP. Owing to the rapid chemical reaction between coke particles and oxygen, the temperature of the sample instantaneously increased. Figure 2 shows a typical result of the temperature variation of the sample. In 60 seconds, temperature monitored with TC-1 reaches a maximum temperature of 1573 K (1300 °C) and then decreases monotonically. Temperature monitored with TC-2 shows a similar variation with a time interval of 40 seconds. From the difference in temperature variations monitored with two thermocouples, the moving velocity of the combustion zone was estimated to be approximately 0.5 mm/s. When the temperature of TC-1 decreased to 1273 K (1000 °C), the electric current applied to the furnace was shut down and the sample was cooled to room temperature in the furnace. Due to the temperature profile obtained in this experimental setup, it was possible to demonstrate the initial stage of the practical sinter process with this experimental facility.
After the experiments, XRD analysis (D8 Advance, Bruker, Cu Kα target, 40 kV, 40 mA), wet-chemical analysis, and SEM-EDX analysis were carried out to identify the phases and compositions of the samples. In XRD analysis, the 2θ range was varied from 20 to 120 deg at the scan speed of 0.8 deg/min. X’Pert HighScore Plus 4.7(Malvern Panalytical, Netherlands) was used to refine the phases and to perform Rietveld analysis.
Results and Discussion
Effect of ZnO
To investigate the behavior of Zn in the iron ore sinters, ZnO content in the raw mixtures was varied from 1 to 3 wt pct (samples 1 through 3), while the basicity was fixed at 2 and the Al2O3 content was 1 wt pct. Figure 3 shows the identification results of XRD patterns of samples 1 through 3, and the comparison between the calculated and measured XRD patterns. At the bottom of each figure, differences between the calculated and measured profiles are shown. Commonly, Fe2O3, Fe3O4, FeO, SFCA, C2S, franklinite, and Al2O3 were identified. No zincite (ZnO) was detected in the present samples. Since the binary system of franklinite and Fe3O4 is isomorphous, the XRD patterns for franklinite and Fe3O4 are almost identical. Therefore, the phase ratio was calculated with the values of lattice parameters of franklinite and Fe3O4.[40] In XRD patterns, various calcium ferrite phases, such as CaFe2O4 (CF),[41] Ca2Fe2O5 (C2F),[42] and CaFe4O7 (CF2),[43] were also detected, but the amount of each phase was less than 0.6 wt pct. Therefore, these phases were excluded from the quantitative analysis. The formation behavior of complex calcium ferrites depends on oxygen partial pressure.[18,38] Among various complex calcium ferrites, SFCA was the main mineral phase. It is considered that high oxygen partial pressure leads to a favorable condition for forming the SFCA. SFCA was generated by the reaction between the liquid phase and magnetite during sintering, as suggested by Webster et al.[19] The Rietveld analysis results are summarized in Table III, and the phase fractions are shown as a function of bulk ZnO content in Figure 4. In this study, the weighted profile R factor (Rwp) and goodness-of-fit values on the Rietveld analysis are within the acceptable ranges of 1.59 to 1.97 and 1.30 to 1.55, respectively. With increasing ZnO content, the phase fractions of SFCA, Fe3O4, C2S, FeO, and franklinite increased, whereas that of Fe2O3 decreased. Chemical analysis results are summarized in Table IV. It can be expected that some amount of ZnO could be reduced by coke during the experiments, but no significant change in ZnO content was noticed in this study. Besides, from the XRD analysis, zincite was not detected. Accordingly, it was considered that ZnO was dissolved in some phases such as franklinite.
To identify each mineral phase, the cross-sectional images were taken by using backscattered electron (BSE) microscopy. Then, SEM-EDX analysis was carried out to investigate the chemical composition of the selected phases. Figure 5 shows the BSE micrographs for samples 1 through 3. It is found that needlelike SFCA, Fe2O3, and Fe3O4 are the dominant phases. FeO, franklinite, and Al2O3 were detected from XRD analysis but are too small to be investigated from the cross-sectional image. From EDX analysis, Zn was detected in SFCA. To obtain the average composition of SFCA, three points of SFCA for each sample were investigated with SEM-EDX. The analyzed points are indicated as green dots in Figure 4. The average composition of SFCA is summarized in Table V. It is found that SFCA contained 1.34 to 2.02 wt pct ZnO. Accordingly, the majority of the increased ZnO resulted in the formation of the franklinite. Since Zn has low boiling temperature and high vapor pressure, it can be easily volatilized during the sinter plant operation under a reducing atmosphere.[6] However, from this study, it was confirmed that ZnO formed franklinite and it was partially dissolved in SFCA at least in the initial stage of the practical sinter operation. It should be noted that the reducing condition was maintained only for 60 seconds, while coke particles reacted with the 79 pct N2 to 21 pct O2 carrier gas mixture, and after consuming coke particles, it was switched to the oxidizing condition. In a conventional sinter plant, reduction and evaporation of Zn and reoxidation may occur simultaneously.[12] To investigate the removal of Zn in a conventional sinter plant, kinetic studies should be carried out separately under isothermal conditions for a longer period.
BSE micrograph of the samples: (a) sample 1 (1 wt pct ZnO), (b) sample 2 (2 wt pct ZnO), and (c) sample 3 (3 wt pct ZnO). S: SFCA, H: hematite, SP: spinel group including magnetite and franklinite, C2S: dicalcium silicate, V: void, and EDX analysis points are indicated as green dots (Color figure online)
Effect of Basicity
To investigate the effect of basicity, the basicity was controlled from 2 to 4, while the ZnO and Al2O3 contents were fixed at 1 wt pct, respectively. For this purpose, samples 1, 4, and 5 are compared. Figure 6 shows the identification results of the XRD patterns of samples 4 and 5. Fe2O3, Fe3O4, FeO, SFCA, C2S, franklinite, and Al2O3 were identified for these samples. The Rietveld analysis results are summarized in Table III and graphically shown in Figure 7. The amount of SFCA increased with increasing basicity, whereas those of Fe2O3, Fe3O4, and franklinite decreased.
Figure 8 shows the BSE microscope observation results of samples 4 and 5. It is found that with increasing basicity, the fraction of SFCA increased from 47.4 to 79.2 wt pct and the width of the columnar SFCA became larger. As the fraction of SFCA increased, the fraction of franklinite decreased from 1.1 to 0.2 wt pct, while ZnO content in the SFCA was almost constant in the range of 1.08 to 1.48 wt pct (Table V). Accordingly, it is considered that as the basicity increased, ZnO tended to be preferentially dissolved in SFCA rather than forming franklinite.
Effect of Al2O3
To investigate the effect of Al2O3, the Al2O3 content in the iron ore sinter was controlled from 1 to 3 wt pct, while the basicity was fixed at 2 and the ZnO content was 1 wt pct. For this purpose, samples 1, 6, and 7 are compared. Figure 9 shows the identification results of the XRD patterns of samples 6 and 7, and the Rietveld analysis results are graphically shown in Figure 10. As the Al2O3 content increased from 1 to 3 wt pct, the amount of SFCA increased from 47.4 to 83.2 wt pct. On the other hand, the amount of Fe2O3, Fe3O4, and franklinite decreased. Al2O3 is known as a stabilizing element for the formation of calcium ferrite,[44,45,46] and Webster et al.[19] reported that with increasing Al2O3 content, the formation temperature of SFCA in the cooling process increased. Accordingly, increased Al2O3 content would accelerate the formation of SFCA in the sinter.
Figure 11 shows the BSE microscope observation results of samples 6 and 7. There is no significant morphological change investigated in these samples. The ZnO content in SFCA of each sample did not change so much with increasing the Al2O3 content, while the fraction of SFCA considerably increased. Accordingly, it is concluded that most of the ZnO is dissolved in the SFCA phase.
Crystal Structure Change of SFCA
In the crystal structure of SFCA, the substitution of atoms is performed between tetragonal sites and octahedral sites. Hamilton et al.[17] reported that substitutions are made in M6, Ca1, and M12 sites. Here, M6 and M12 are the tetrahedral sites and Ca1 is the octahedral site. Figure 12 shows the positions of the atoms in the crystal structure. They assumed that M6 is occupied by Al, while M12 is substituted between Si and Al, and Ca1 is substituted between Ca and Fe. In this study, for the first time, it can be assumed that Zn substitutes the tetragonal sites as well as the octahedral sites. The probable position of the Zn atom is then simulated by placing the Zn atom in each site using a simulation program, X’pert highs score. The simulation results are summarized in Table VI. The atomic coordinate information of the substitution site, which is used in the simulation and reported by Hamilton et al.,[17] is indicated in Table VII. The simulation results show that the most probable position of Zn is the M12 sites. Takayama et al.[24] reported that Fe can be substituted not only at Ca1 sites but also at M6 sites. In this study, to satisfy the mass balance of Fe in SFCA, it was optimized that Fe substituted at all M6, Ca1, and M12 sites.
Figure 13 shows the composition change in SFCA obtained from EDX analysis and the change of the atomic occupancy at M6, Ca1, and M12 sites as ZnO content in sinter increases. As the initial ZnO content increases, the concentrations of ZnO and Al2O3 in SFCA increase, whereas that of SiO2 decreases. Structural analysis reveals that Fe mostly occupies M6 and Ca1 sites. On the other hand, Si, Zn, and Al occupy M12 sites, where the substitution occurs. With increasing ZnO content, Zn and Al occupancies increase, whereas Si occupancy decreases.
Figure 14 shows the composition change in SFCA and the change of the atomic occupancy at M6, Ca1, and M12 sites as basicity in sinter increases. As the basicity increases, the concentration of Fe2O3 increases, while that of SiO2 decreases. This trend is also related to the increased occupancy of Fe at the M12 sites and the decreased occupancy of Si. However, there is no significant change in the Zn occupation at the M12 sites.
Figure 15 shows the composition change in SFCA and the change of the atomic occupancy at M6, Ca1, and M12 sites as Al2O3 content in sinter increases. As the initial Al2O3 content increases from 1 to 3 wt pct, the concentration of Al2O3 in the SFCA increases from 1.30 to 5.29 wt pct, whereas that of Fe2O3 decreases from 80.46 to 71.85 wt pct. This trend is related to the increased occupancy of Al and the decreased occupancy of Fe in the M6 sites. However, it is noticeable that atomic occupancy at the M12 sites does not change considerably. The atomic occupancy at the Ca1 sites is relatively complex. Nevertheless, the occupancy of Zn is investigated at the M12 sites only, and it did not change with increasing Al2O3 content.
Based on the preceding simulation results, it is concluded that when ZnO is added in the sinter, it is dissolved in the SFCA phase and Zn preferentially occupies the M12 site. It is also found that the basicity and content of Al2O3 do not affect the dissolution behavior of Zn in the SFCA phase. Since the Rietveld analysis results are comparable with the SEM-EDX analysis results, it is quite reasonable to accept the simulation results.
Conclusions
As the use of by-products increases in the iron ore sinter process, it is necessary to understand the phase stability of the iron ore sinter containing ZnO. In this study, the effects of the ZnO addition, basicity, and Al2O3 content on the behavior of Zn in the iron ore sinter were investigated. The phase fractions, the composition of SFCA, and the substitution sites of Zn could be identified by using Rietveld analysis, wet-chemical analysis, and SEM-EDX analysis. It was confirmed that ZnO existed as franklinite or was dissolved in the SFCA phase of the iron ore sinter. As the ZnO content increased, the amount of franklinite and SFCA increased, while the amount of Fe2O3 decreased. It was considered that Zn occupies the M12 sites of the SFCA phase. As the basicity of the iron ore sinter increased, the amount of franklinite considerably decreased, while the amount of SFCA increased. Here, the occupancy rate of Zn in the M12 sites did not change so much in the SFCA phase. As the Al2O3 content increased, the amount of franklinite slightly decreased and the amount of SFCA remarkably increased. Here, the occupancy rate of Zn in the M12 sites remained almost constant. Accordingly, with increasing Al2O3, the SFCA containing ZnO became more stable. From this study, it is concluded that to remove Zn from the iron ore sinter, a kinetic study on the chemical reaction between CO and Zn in the SFCA is required in the future.
References
1. H.T. Makkonen, J. Heino, L. Laitila, A. Hiltunen, E. Poylio, and J. Harkki: Resources Conserv. Recy., 2002, vol. 35, pp. 77–84.
2. D. Hleis, I. Fernandez-Olmo, F. Ledoux, A. Kfoury, L. Courcot, T. Desmonts, and D. Courcot: J. Hazard. Mater., 2013, vols. 250–251, pp. 246–55.
3. N.C.C. Lobato, E.A. Villegas, and M.B. Mansur: Resources Conserv. Recy., 2015, vol. 102, pp. 49–57.
4. C. Lanzerstorfer: ISIJ Int., 2017, vol. 57, pp. 1484–89.
B. AsadiZeydabadi, D. Mowla, M.H. Shariat, and J. FathiKalajahi: Hydrometallurgy, 1997, vol. 47, pp. 113–25.
6. C. Lanzerstorfer, B. Bamberger-Strassmayr, and K. Pilz: ISIJ Int., 2015, vol. 55, pp. 758–64.
7. W. Lv, M. Gan, X. Fan, Z. Ji, X. Chen, J. Yao, and T. Jiang: JOM, 2019, vol. 71, pp. 3173–80.
8. D.E. Esezobor and S.A. Balogun: Ironmak. Steelmak., 2006, vol. 33, pp. 419–25.
9. P. Besta, K. Janovska, A. Samolejova, A. Berankova, I. Voznakova, and M. Hendrych: Metalurgija, 2013, vol. 52, pp. 197–200.
10. K. Narita, T. Onoye, Y. Satoh, M. Miyamoto, K. Taniguchi, S. Kamatani, T. Sato, and S. Fukihara: Trans. ISIJ, 1981, vol. 21, pp. 839–45.
11. J. Wang, Z. Wang, Z. Zhang, and G. Zhang: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 480–90.
H. Li, D.J. Pinson, P. Zulli, L. Lu, R.J. Longbottom, S.J. Chew, B.J. Monaghan, and G. Zhanga: J. Hazard. Mater., 2020, vol. 385, 121592
13. H. Ishikawa, J. Kopfle, J. McClelland, and J. Ripke: Arch. Metall. Mater., 2008, vol. 53 (2), pp. 541–45.
14. R.A. Shawabkeh: Hydrometallurgy, 2010, vol. 104, pp. 61–65.
15. A. Greco, D. Jeulin, and J. Serra: J. Microsc., 1979, vol. 116, pp. 199–211.
16. Z. Wang, D. Pinson, S. Chew, B.J. Monaghan, M.I. Pownceby, N.A.S. Webster, H. Rogers, and G. Zhang: ISIJ Int., 2016, vol. 56, pp. 1138–47.
17. J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, and M.A. Montague: Neues Jahrb. Mineral. Abh., 1989, vol. 161, pp. 1–26.
18. N.A.S. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: ISIJ Int., 2013, vol. 53, pp. 774–81.
19. N.A.S. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1344–57.
20. N.A.S. Webster, M.I. Pownceby, and I.C. Madsen: ISIJ Int., 2013, vol. 53, pp. 1334–40.
21. N.A.S. Webster, M.I. Pownceby, I.C. Madsen, A.J. Studer, J.R. Manuel, and J.A. Kimpton: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 2097–2105.
22. N.A.S. Webster, M.I. Pownceby, R. Pattel, J.R. Manuel, and J.A. Kimpton: ISIJ Int., 2019, vol. 59, pp. 263–67.
23. N.A.S. Webster, M.I. Pownceby, R. Pattel, J.R. Manuel, and J.A. Kimpton: ISIJ Int., 2019, vol. 59, pp. 1007–10.
24. T. Takayama, R. Mura, and M. Kimura: ISIJ Int., 2018, vol. 58, pp. 1069–78.
25. J. Hancart, V. Leroy, and A. Bragard: CNRM Metall. Rep., 1967, No. 11, pp. 3–7.
26. K. Inoue and T. Ikeda: Tetsu-to-Hagané, 1982, vol. 68, pp. 2190–99.
27. P.R. Dawson, J. Ostwald, and K.M. Hayes: BHP Tech. Bull., 1983, vol. 27, pp. 47–51.
28. T.R.C. Patrick and M.I. Pownceby: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 79–89.
29. R. Murao, T. Harano, M. Kimura, and I.-H. Jung: ISIJ Int., 2018, vol. 58, pp. 259–66.
30. S. Nicol, J. Chen, M.I. Pownceby, and N.A.S. Webster: ISIJ Int., 2018, vol. 58, pp. 2157–72.
31. H. Guo and X.-M. Guo: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 1974–84.
32. J. Chen, M. Shevchenko, P.C. Hayes, and E. Jak: ISIJ Int., 2019, vol. 59, pp. 795–804.
33. T.J. Park, J.S. Choi, and D.J. Min: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2174–81.
34. T.J. Park, J.S. Choi, and D.J. Min: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 790–98.
35. S. Nicol, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2706–22.
36. S. Nicol, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 3027–38.
37. K. Taira and M. Matsumura: ISIJ Int., 2018, vol. 58, pp. 808–14.
38. J.W. Jeon, S.M. Jung, and Y. Sasaki: ISIJ Int., 2010, vol. 50, pp. 1064–70.
39. J. Chen, M. Shevchenko, P.C. Hayes, and E. Jak: ISIJ Int., 2019, vol. 59, pp. 805–09.
40. S.C. Schaefer and R.A. McCune: Metall. Mater. Trans. B, 1986, vol. 17B, pp. 515–21.
41. B.F. Decker and J.S. Kasper: Acta Crystallogr., 1957, vol. 10, pp. 332–37.
42. F. Bertaut, P. Blum, and A. Sagnieres: C. R. Acad. Sci., 1957, vol. 244, pp. 2944–46.
43. E. Millon, B. Malaman, A. Bonazebi, J.F. Brice, R. Gerardin, and O. Evrard: Mater. Res. Bull., 1986, vol. 21, pp. 985–94.
45. D.Y. Lee, H.K. Shin, S.M. Moon, W.B. Chung, and W.S. Chung: J. Kor. Inst. Met. Mater., 1996, vol. 34, pp. 387–96.
46. J.H. Park, Y.J. Cho, S.S. Yoon, W.W. Huh, and H.S. Kim: J. Kor. Inst. Met. Mater., 2002, vol. 40, pp. 811–17.
47. S.J. Yoon: J. Kor. Inst. Met. Mater., 2006, vol. 44, pp. 70–76.
Acknowledgments
This research was funded and conducted under “the Competency Development Program for Industry Specialists” of the Korean Ministry of Trade, Industry, and Energy (MOTIE), operated by the Korea Institute for Advancement of Technology (KIAT) (Grant No. P0002019, HRD Program for High Value-Added Metallic Material Expert) and POSCO (POSCO research Grant).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted March 24, 2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, J., Rajavaram, R., Suh, IK. et al. Effects of Basicity and Al2O3 Content on the Chemistry of Phases in Iron Ore Sinter Containing ZnO. Metall Mater Trans B 51, 3016–3027 (2020). https://doi.org/10.1007/s11663-020-01940-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01940-7



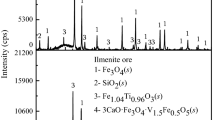




 : Fe2O3,
: Fe2O3,  : Fe3O4,
: Fe3O4,  : SFCA,
: SFCA,  : C2S,
: C2S,  : FeO,
: FeO,  : franklinite, and
: franklinite, and  : Al2O3. At the bottom of each figure, differences between the calculated and measured profiles are shown
: Al2O3. At the bottom of each figure, differences between the calculated and measured profiles are shown










