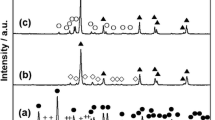
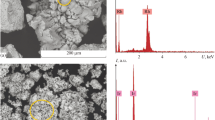
Abstract
Acids such as aqua regia, a 3:1 mixture of hydrochloric acid (HCl) and nitric acid, that contain strong oxidizing agents are used in the dissolution-based recovery of platinum group metals (PGMs). However, nitrate-nitrogen emission has become subject to increasingly strict environmental regulations in recent years. Accordingly, we herein propose a dissolution process for PGMs via the formation of complex oxides using HCl alone. We prepared complex oxides of alkali metals and rhodium (Rh), which is particularly hard to dissolve, and investigated their dissolution behaviors in HCl. Rh-containing complex oxides were prepared by calcining Rh powder and alkali metal salts in air, and dissolution tests using HCl were conducted on the complex oxides obtained. It was found that Rh-containing complex oxides were completely dissolved under appropriate calcination and dissolution conditions.










Similar content being viewed by others
References
J. Zhang, M.P. Everson, T.J. Wallington, F.R. Field, R. Roth, and R.E. Kirchain: Environ. Sci. Technol., 2016, vol. 50, pp. 7687−7695.
Thomson Reuters: GFMS Platinum Group Metals Survey 2018, 2018.
E. Kizilaslan, S. Aktaş, and M.K. Şeşen: Turkish J. Eng. Env. Sci., 2009, vol. 33, pp. 83–90.
M.K. Jha, J.-C. Lee, M.-S. Kim, J. Jeong, B.-S. Kim, and V. Kumar: Hydrometallurgy, 2013, vol. 133, pp. 23–32.
K. Fujita, H. Onoue, and K. Sakiyama: Corros. Eng. (Boshoku-Gijutsu), 1970, vol. 19, pp. 340−345 (in Japanese).
World Health Organization: Guidelines for Drinking-Water Quality, 4th ed., 2011, Chapter 8 Chemical aspects, pp. 155–201.
Online document: Ministry of the Environment, Environmental quality standards for human health. http://www.env.go.jp/en/water/wq/wp.pdf. Accessed 9 November 2018
Online document: Ministry of the Environment, National Effluent Standards. https://www.env.go.jp/en/water/wq/nes.html, 2015. Accessed 9 November 2018.
Online document: Ministry of the Environment, Ministerial Ordinance of the Effluent Standards. http://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=346M50000002035#120, 2016. Accessed 9 November 2018.
Y. Kayanuma, S. Mizuhashi, and Y. Shindo: J. Jpn. Inst. Met. Mater., 2017, vol 4, pp. 152–156 (in Japanese).
Organization site: Code of Federal Regulations, Title 40 Protection of Environment, https://www.ecfr.gov/. Accessed 11 January, 2019.
Organization site: Code of Federal Regulations, Title 40 Protection of Environment, Part 421, Nonferrous Metals Manufacturing Point Source Category, Retrieved from https://www.ecfr.gov/. Accessed 11 January, 2019.
European Communities: Off. J. Eur. Commun., 1991, vol. L135, pp. 1–13.
European Communities: Off. J. Eur. Commun., 1991, vol. L375, pp. 1–8.
J.E. Barnes and J.D. Edwards: Chem. Ind., 1982, vol. 5, pp. 151–155.
T.H. Okabe and K. Nose: The Latest Technological Trend of Rare Metals, CMC Publishing Co., Ltd., Tokyo, 2012, pp. 76–84 (in Japanese).
Y. Cao, A. Shibayama, S. Harjanto, I. Naitoh, T. Nanami, T. Kasahara, and T. Fujita: Trans. Soc. Automotive Eng. Jpn., 2007, vol. 38, pp. 55–61.
. W. Westwood: Platinum Supplement, Gmelin Handbook of Inorganic Chemistry, 8th Ed., Springer-Verlag, 1985, vol. A1, pp. 30−32.
K. Gloe, P. Mühl, and M. Knothe: Hydrometallurgy, 1990, vol. 25, pp. 99−110.
M. M. Totland, I. Jarvis, and K. E. Jarvis: Chem. Geol., 1995, vol. 124, pp. 21−36.
J. Lee and Y. Kim: Mater. Trans., 2011, vol. 52, pp. 2067−2070.
K. J. De Vries and P. J. Gellings: J. Inorg. Nucl. Chem., 1969, vol. 31, pp. 1307−1313.
T. H. Okabe, Y. Kayanuma, S. Yamamoto and M. Maeda: Mater. Trans., 2003, vol. 44, pp. 1386−1393.
K. Nomura, M. Daté, H. Kageyama, and S. Tsubota, J. Mater. Res., 2007, vol. 22, pp. 2647−2650.
M. Daté, K. Nomura, H. Kageyama, and T. Fujitani, ChemPhysChem, 2011, vol. 12, pp. 109−111.
R. Kasuya, T. Miki, and Y. Tai: J. Ceram. Soc. Jpn., 2013, vol. 121, pp. 261–264.
R. Kasuya, T. Miki, H. Morikawa, and Y. Tai: J. Ceram. Soc. Jpn., 2013, vol. 121, pp. 884–890.
R. Kasuya, T. Miki, H. Morikawa, and Y. Tai: Int. J. Miner. Process., 2014, vol. 128, pp. 33–39.
R. Kasuya, T. Miki, H. Morikawa, and Y. Tai: Miner. Eng., 2015, vol. 76, pp. 135–140.
R. Kasuya, T. Miki, H. Morikawa, and Y. Tai: Metal. Mater. Trans. B, 2015, vol. 46, pp. 2476–2483.
R. Kasuya, T. Miki, H. Morikawa, and Y. Tai: Miner. Eng., 2016, vol. 87, pp. 25–31.
F. Izumi and K. Momma: Solid State Phenom., 2007, vol. 130, pp. 15–20.
A. Mendiboure, H. Eickenbusch, R. Schöllhorn, and G. V. Subba Rao: J. Solid State Chem., 1987, vol. 71, pp. 19–28.
R. W. G. Wyckoff: Crystal Structures, 2nd ed., Interscience Publishers, New York, 1963, vol. 1, pp. 7–83.
. S.H. Yao, B.B. Zhang, J. Zhou, Y.B. Chen, S.T. Zhang, Z.B. Gu, S.T. Dong, and Y.F. Chen: AIP Advances, 2012, vol. 2, pp. 042140/1−7.
K.T. Jacob, and Y. Waseda: J. Solid State Chem., 2000, vol. 150, pp. 213–220.
H. Müller-Buschbaum: Z. Anorg. Allg. Chem., 2007, vol. 633, pp. 1289–1306 (in German).
. K. Hobbie and R. Hoppe: Z. Anorg. Allg. Chem., vol. 535, pp. 20–30 (in German).
Y. Okamoto, S. Niitaka, M. Uchida, T. Waki, M. Takigawa, Y. Nakatsu, A. Sekiyama, S. Suga, R. Arita, and H. Takagi: Phys. Rev. Lett., 2008, vol. 101, pp. 086404
Y. Luo, C. Cao, B. Si, Y. Li, J. Bao, H. Guo, X. Yang, C. Shen, C. Feng, J. Dai, G. Cao, and Z. Xu: Phys. Rev. B, 2013, vol. 87, pp. 161121.
K.T. Jacob, D. Prusty, and G.M. Kale: J. Alloy. Compd., 2012, vol. 513, pp. 365–372.
K. Yamaura, Q. Huang, M. Moldovan, D.P. Young, A. Sato, Y. Baba, T. Nagai, Y. Matsui, and E. Takayama-Muromachi: Chem. Mater., 2005, vol. 17, pp. 359–365.
V. Todorova and M. Jansen: Z. Anorg. Allg. Chem., 2011, vol. 637, pp. 37–40.
K. Momma and F. Izumi: J. Appl. Crystallogr., 2011, vol. 44, pp. 1272–1276.
G. Brauer: Handbook of Preparative Inorganic Chemistry, 2nd ed., Academic Press, New York, 1965, pp. 1587.
F.L. Bernardis, R.A. Grant, and D.C. Sherington: React. Funct. Polym., 2005, vol. 65, pp. 205–217.
G. Levitin, and G. Schmuckler: React. Funct. Polym., 2003, vol. 54, pp. 149–154.
D.C. Harris: Quantitative Chemical Analysis, 7th ed., W. H. Freeman and Company, New York, 2007, pp. AP20–AP27.
D. R. Lide: CRC Handbook of Chemistry and Physics, CRC Press: Boca Raton, FL, 2005.
Z. Marczenko and M. Balcerzak: Separation, Preconcentration and Spectrophotometry in Inorganic Analysis, 2000, Analytical Spectroscopy Library, vol. 10, Chapter 25: Iodine.
H. Narita, T. Suzuki, and R. Motokawa: J. Jpn. Inst. Met. Mater., 2017, vol. 81, pp. 157–167 (in Japanese).
H. Narita, K. Morisaku, and M. Tanaka: Chem. Commun., 2008, vol. 45, pp. 5921–5923.
H. Narita, K. Morisaku, and M. Tanaka: Solvent Extr. Ion. Exch., 2015, vol. 33, pp. 407–417.
Acknowledgments
This study was supported by a Grant from the Environment Research and Technology Development Fund (3K163010) of the Environmental Restoration and Conservation Agency (ERCA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted May 30, 2019.
Rights and permissions
About this article
Cite this article
Kasuya, R., Nomura, K. & Narita, H. Solubilization of Rhodium in Hydrochloric Acid Using an Alkali Metal Salt Method. Metall Mater Trans B 51, 377–385 (2020). https://doi.org/10.1007/s11663-019-01740-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01740-8