Abstract
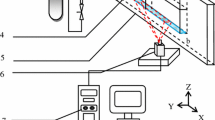
The anodic bubbles generated in aluminum electrolytic cells play a complex role to bath flow, alumina mixing, cell voltage, heat transfer, etc., and eventually affect cell performance. In this paper, the bubble dynamics beneath the anode were observed for the first time from bottom view directly in a similar industrial electrolytic environment, using a laboratory-scale transparent aluminum electrolytic cell. The corresponding cell voltage was measured simultaneously for quantitatively investigating its relevance to bubble dynamics. It was found that the bubbles generated in many spots that increased in number with the increase of current density; the bubbles grew through gas diffusion and various types of coalescences; when bubbles grew to a certain size with their surface reaching to the anode edge, they escaped from the anode bottom suddenly; with the increase of current density, the release frequency increases, and the size of these bubbles decreases. The cell voltage was very consistent with bubble coverage, with a high bubble coverage corresponding to a higher cell voltage. At low current density, the curves of voltage and coverage fluctuated in a regularly periodical pattern, while the curves became more irregular at high current density. The magnitude of voltage fluctuation increased with current density first and reached a maximum value at current density of 0.9 A/cm2, and decreased when the current density was further increased. The extra resistance induced by bubbles was found to increase with the bubble coverage, showing a similar trend with published equations.












Similar content being viewed by others
Abbreviations
- t :
-
Time, s
- a :
-
Anode length, mm
- b :
-
Anode width, mm
- i :
-
Bubble serial ID, dimensionless
- I :
-
Total bubble number in one image, dimensionless
- S i :
-
The ith bubble area, mm2
- j :
-
Image serial ID, dimensionless
- J :
-
Total image number at a fixed current density
- m :
-
Cell voltage datum serial ID, dimensionless
- M :
-
The total number of cell voltage data, dimensionless, dimensionless
- V m :
-
The mth cell voltage datum, V
- V ave :
-
The average value of cell voltage, V
- V max-fluctuation :
-
The maximum fluctuation of cell voltage, V
- V ave-fluctuation :
-
The mean fluctuation of cell voltage, V
- K 0 :
-
The resistivity of the cryolite, ohm m
- d 0 :
-
The depth of the bubble layer, mm
- A :
-
The area of the underside surface of anode, mm2
- H :
-
The depth of ACD, mm
- ΔR :
-
The extra resistance due the existing bubble, ohm
- \( {\varphi } \) :
-
Gas coverage
- \( \overline{\varphi } \) :
-
Average gas coverage
References
K. Grjotheim: Aluminum Electrolysis: Fundamentals of the Hall-Heroult process, 2nd ed, p. 443, Aluminum-Verlag, Dusseldorf, 1982.
S. Fortin, M. Gerhardt, and A. J. Gesing: Light Metals, TMS, Los Angeles, PA, 1984, pp. 385–95.
W. E. Haupin: JOM, 1971, vol. 23(10), pp. 46–49.
W. E. Haupin, and W. C. McGrew: Aluminium, 1975, vol. 51, pp. 273–75.
N. E. Richards: Light Metals, TMS, Phoenix, PA, 1988, pp. 521–29.
N. Richards, H. Gudbrandsen, S. Rolseth, and J. Thonstad: Light Metals, TMS, San Diego, PA, 2003, pp. 315–22.
W. Zhang: Modeling of Anode Gas Evacuation and Current Efficiency in Hall-Heroult cells, University of Auckland, Auckland, 1993.
K. Qian, Z. D. Chen, and J. J. J. Chen: J. Appl. Electrochem., 1998, vol. 28, pp. 1141–45.
S. Poncsak, L. I. Kiss, D. Toulouse, A. Perron, and S. Peron: Light Metals, TMS, San Antonio, PA, 2006, pp. 457–62.
Y. Wang, L. Zhang, and X. Zuo: Light Metals, TMS, San Francisco, PA, 2009, pp. 581–86.
Y. Wang, and L. Zhang: Light Metals, TMS, Warrendale, PA, 2010, pp. 207–14.
K. Vekony, and L. I. Kiss: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 1006–17.
K. Vekony, and L. I. Kiss: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1086–97.
X. Wang, G. Tarcy, S. Whelan, S. Porto, C. Ritter, and B. Ouellet: Light Metals, TMS, Orlando, PA, 2007, pp. 539–44.
S. Das, Y. Morsi, G. Brooks, W. Yang, and J.J.J. Chen: Colloids Surf. A, 2012, vol. 411, pp. 94–104.
K. Zhang, Y. Feng, P. J. Witt, W. Yang, M. Cooksey, Z. Wang, and M. P. Schwarz: J. Appl. Electrochem., 2014, vol. 44, pp. 1081–92.
K. Qian, and J. J. J. Chen: J. Appl. Electrochem., 1997, vol. 27, pp. 434–40.
J. J. J. Chen, K. X. Qian, and J. C. Zhao: Trans IChemE, 2001, vol. 79, 383–88.
Y. Xue, N. Zhou, and S. Bao: Chin. J. Nonferrous. Met., 2006, vol. 16, pp. 1823–28.
M. Alam, W. Yang, K. Mohanarangam, G. Brooks, and Y. S. Morsi: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 1155–65.
K. Zhang, Y. Feng, M. P. Schwarz, M. Cooksey, and Z. Wang: Light Metals, TMS, Warrendale, PA, 2012, pp. 881–86.
K. Zhang, Y. Feng, M. P. Schwarz, Z. Wang, and M. Cooksey: Ind. Eng. Chem. Res., 2013, vol. 52, pp. 11378–390.
Z. Qiu, L. Fan, and K. Grjotheim: Light Metals, TMS, New Orleans, PA, 1986, pp. 525–33.
Z. Qiu, L. Fan, N. Feng, K. Grjotheim, and H. Kvande: Light Metals, TMS, Colorado, PA, 1987, pp. 409–16.
Z. Qiu, and M. Zhang: Electrochimica Acta., 1987, vol. 32, pp. 607–13.
Z. Qiu, L. Fan, K. Grjotheim, and H. Kvade: J. Appl. Electrochem., 1987, vol. 17, pp. 707–14.
B. Gao, X. Hu,J. Xu, Z. Shi, Z. Wang, and Z. Qiu: Light Metals, TMS, San Antonio, PA, 2006, pp. 467–70.
J. Xu, Z. Shi, B. Gao, and Z. Qiu: Chin. J. Nonferrous. Met., 2004, vol. 14, pp. 298–301.
S. Yang, F. Yang, Q. Liu, X. Hu, Z. Wang, Z. Shi, and B. Gao: Light Metals, TMS, San Francisco, PA, 2009, pp. 65–68.
T. Utigard, and J. M. Toguri: Light Metals, TMS, New Orleans, PA, 1986, pp. 405–13.
T. Utigard, J. M. Toguri, and S. W. Ip: Light Metals, TMS, Phoenix, PA, 1988, pp. 703–06.
L. Cassayre, T. A. Utigard, and S. Bouvet, S: JOM, 2002, vol. 54(5), pp. 41–45.
L. Cassayre, G. Plascencia, T. Marin, S. Fan, and T. A. Utigard: Light Metals, TMS, San Antonio, PA, 2006, pp. 379–383.
J. Xue, and H. A. Oye: Light Metals, TMS, Las Vegas, PA, 1995, pp. 265–71.
Z. Zhao, Z. Wang, B. Gao, Y. Feng, Z. Shi, and X. Hu: Light Metals, TMS, Orlando, PA, 2015, pp. 801–06.
N. Feng: Aluminum Electrolysis, 1st ed., p. 401, Chemical industry press, Beijing, 2006.
Z. Qiu: Principle and Application of Aluminum Electrolysis, 1st ed., p. 572, China University of Mining and Technology Press, Xuzhou, 1998.
R. J. Aaberg, V. Ranum, K. Willisamson, and B. J. Welch: Light Metals, TMS, Warrendale, PA, 1997, pp. 341–46.
Hartland, Stanley and R. Whitham: Axisymmetric Fluid-Liquid Interfaces: Tables Giving the Shape of Sessile and Pendant Drops and External Menisci, with Examples of their Use, 1st ed. Elsevier Scientific Publishing Co., New York, 1976, p. 782.
P. Aussillous, and D. Quere: Europhys. Lett., 2002, vol. 59, pp. 370–376.
S. Poncsak, L. I. Kiss, R. T. Bui, P. Desclaux, J. P. Huni, and V. Potocnik: Light Metals, TMS, Nashville, PA, 2000, pp. 139–54.
M. A. Cooksey, M. P. Taylor, and J. J. J. Chen: JOM, 2008, vol. 60, pp. 51–57.
P. J. Side, and C. W. Tobias: J. Electrochem. Soc., 1982, vol. 129(12), pp. 2715–20.
G. J. Houston, M. P. Taylor, and D. J. Williams: Light Metals, TMS, Phoenix, PA, 1988, pp. 641–45.
A. Solheim and J. Thonstad: Light Metals, TMS, New Orleans, PA, 1986, pp. 397–403.
Acknowledgements
The authors would like to express their gratitude for the financial support by the National Natural Science Foundation of China (Grant Nos. 51322406, 51434005, 51474060, 51574070, and 51529401). Zhibin Zhao would like to thank the China Scholarship Council (CSC) for a visiting PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 12, 2015.
Rights and permissions
About this article
Cite this article
Zhao, Z., Wang, Z., Gao, B. et al. Anodic Bubble Behavior and Voltage Drop in a Laboratory Transparent Aluminum Electrolytic Cell. Metall Mater Trans B 47, 1962–1975 (2016). https://doi.org/10.1007/s11663-016-0598-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0598-9