Abstract
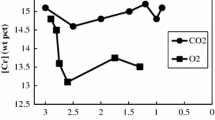
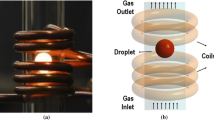
The influence of thermal diffusion on the kinetics of decarburization of Fe-Cr-C droplets with CO2-Ar gas mixtures was investigated. With incorporation of the effect of thermal diffusion, a new correlation has been proposed to express the decarburization kinetics of levitated droplets for flows in the range of Reynolds number between 2 and 100. A thermal diffusion factor of 0.228 was evaluated for CO2-Ar gas mixtures at 1873 K (1600 °C).




Similar content being viewed by others
Abbreviations
- A :
-
Area of droplet surface (cm2)
- Ar:
-
Archimedes number \( \left({ = \frac{{{\text{Gr}}^{\prime}}}{{{\text{Re}}^{2} }}} \right) \)
- C :
-
Total molar concentration in gas phase (mol cm−3)
- C p :
-
Heat capacity of gas (J g−1 K−1)
- D AB :
-
Mutual diffusion coefficient in gas phase (cm2 s−1)
- D T :
-
Thermal diffusion coefficient (cm2 s−1)
- d p :
-
Diameter of the droplet (cm)
- Gr′:
-
Mean Grashof number \( \left( { = {\text{Gr}}_{\text{m}} + {\text{Gr}}_{\text{H}} \left( {{\text{Sc}}/{ \Pr }} \right)^{0.5} } \right) \)
- Grm :
-
Grashof number for mass transfer \( \left( { = \frac{{\rho_{\text{g}} gd_{\text{p}}^{3} \left( {C_{i} - C_{\text{b}} } \right)}}{{\mu_{\text{g}}^{2} }}} \right) \)
- GrH :
-
Grashof number for heat transfer \( \left( { = \frac{{gd_{\text{p}}^{3} \left( {T_{i} - T_{\text{b}} } \right)}}{{T_{\text{f}} \mu_{\text{g}}^{2} }}} \right) \)
- h :
-
Heat transfer coefficient (J cm−1 s−1 K−1)
- J i :
-
Flux of diffusion species i (m cm−2 s−1)
- k :
-
Thermal conductivity of gas (J cm−1 s−1 K−1)
- k T :
-
Thermal diffusion ratio
- m :
-
Coefficient in Eq. [11]
- n :
-
Coefficient in Eq. [11]
- \( {\text{Nu}} \) :
-
Nusselt number \( \left( { = \frac{{d_{\text{p}} h}}{k}} \right) \)
- P :
-
Total pressure (atm)
- Pr:
-
Prandtl number \( \left( { = \frac{{\mu_{\text{g}} C_{\text{p}} }}{k}} \right) \)
- R :
-
Gas constant (cm3 atm mol−1 K−1)
- r:
-
Radial distance from the droplet surface (cm)
- Re:
-
Reynolds number \( \left( { = \frac{{d_{\text{p}} v\rho_{\text{g}} }}{{\mu_{\text{g}} }}} \right) \)
- Sc:
-
Schmidt number \( \left( { = \frac{{\mu_{\text{g}} }}{{\rho_{\text{g}} D_{\text{AB}} }}} \right) \)
- Sh:
-
Sherwood number \( \left( { = \frac{{d_{\text{p}} k_{\text{g}} }}{{D_{\text{AB}} }}} \right) \)
- t :
-
Time (s)
- T E :
-
Effective temperature of gases (K) \( \left( { = 0.83\frac{{T_{i} + T_{\text{b}} }}{2}} \right) \)
- T f :
-
Film temperature of gases (K) \( \left( { = \frac{{T_{i} + T_{\text{b}} }}{2}} \right) \)
- T b :
-
Bulk gas temperature (K)
- T i :
-
Gas–metal interface temperature (K)
- v :
-
Relative velocity between gas and droplet (cm s−1)
- W :
-
Mass of the droplet (g)
- \( X_{{{\text{CO}}_{2} }}^{\text{b}} \) :
-
Mole fraction of CO2 in the bulk gas
- \( X_{{{\text{CO}}_{2} }}^{i} \) :
-
Mole fraction of CO2 on the gas–metal interface
- x i :
-
Mole fraction of component i
- α :
-
Binary thermal diffusion factor
- α i :
-
Thermal diffusion factor of component i
- β :
-
Coefficient in Eq. [11]
- σ SB :
-
Stefan–Boltzmann constant \( ( = 5.67037 \times 10^{ - 12} \,{\text{J}}\,{\text{cm}}^{ - 2} \,{\text{s}}^{ - 1} \,{\text{K}}^{ - 4} ) \)
- ɛ :
-
Emissivity of metal
- μ g :
-
Gas viscosity (g cm−1 s−1)
References
P. Wu, Y. Yang, M. Barati and A. McLean: Metall. Mater. Trans. B, 2014, DOI 10.1007/s11663-014-0126-8.
W.E. Ranz and W.R. Marshall: Chem. Eng. Prog., 1952, vol. 48, pp. 141-146.
R.L. Steinberger and R.E. Treybal: AIChE J., 1960, vol. 6 (2), pp. 227-232.
D.R. Sain and G.R. Belton: Metall. Trans. B, 1976, vol. 7B, pp. 235-244.
M. Sunderland, A.E. Hamielec, W.K. Lu and A. McLean: Metall. Trans. B, 1973, vol. 4B, pp. 575-583.
N. El-Kaddah and J. Szekely: Metall. Trans. B, 1983, vol. 14B, pp. 401-410.
A.G. Shashkov, A.F. Zolotukhina, T.N. Abramenko, B.P. Mathur and S.C. Saxena: J. Phys. B: At. Mol. Phys., 1979, vol. 12 (21), pp. 3619-3630.
F.R.W. McCourt: Mol. Phys., 2003, vol. 101 (21), pp. 3223-3229.
W.L. Taylor and P.T. Pickett: Int. J. Thermophys., 1986, vol. 7 (4), pp. 837-849.
N. Frössling: Beitraege zur Geophysik, 1938, vol. 52, pp. 170-216.
H.G. Lee and Y.K. Rao: Metall. Trans. B, 1982, vol. 13B, pp. 403-409.
H. Sun and R.D. Pehlke: Metall. Mater. Trans. B, 1995, vol. 26B, pp. 335-344.
Appreciation is expressed to the Natural Sciences and Engineering Research Council of Canada for provision of funding in support of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 24, 2014.
Appendix
Appendix
Assuming an ideal gas phase, the density is calculated from the relationship:
Data for viscosity, inter-diffusivity, heat capacity, and thermal conductivity are available from AspenONE Engineering Suite – Heat Exchanger Design.
CO2 mol fraction | ρ g, Density at T f | μ g, Viscosity at T f | C p, Heat capacity at T f | k, Thermal conductivity of gas at T f |
|---|---|---|---|---|
T f = 1079 K (806 °C); D AB = 1.520 cm2 s−1 | ||||
2 | 4.52 × 10−4 | 5.58 × 10−4 | 0.532 | 4.60 × 10−4 |
6 | 4.54 × 10−4 | 5.61 × 10−4 | 0.560 | 4.64 × 10−4 |
10 | 4.56 × 10−4 | 5.52 × 10−4 | 0.600 | 4.61 × 10−4 |
15 | 4.58 × 10−4 | 5.60 × 10−4 | 0.636 | 4.62 × 10−4 |
20 | 4.60 × 10−4 | 5.37 × 10−4 | 0.679 | 4.64 × 10−4 |
25 | 4.63 × 10−4 | 5.29 × 10−4 | 0.717 | 4.70 × 10−4 |
30 | 4.65 × 10−4 | 5.22 × 10−4 | 0.755 | 4.82 × 10−4 |
CO2 mol fraction | ρ g, Density at T E | μ g, Viscosity at T E | C p, Heat capacity at T E | k, Thermal conductivity of gas at T E |
|---|---|---|---|---|
T E = 896 K (623 °C); D AB = 1.247 cm2 s−1 | ||||
10 | 5.47 × 10−4 | 4.90 × 10−4 | 0.594 | 3.93 × 10−4 |
30 | 5.58 × 10−4 | 4.62 × 10−4 | 0.738 | 4.11 × 10−4 |
Rights and permissions
About this article
Cite this article
Wu, P., Yang, Y., Barati, M. et al. The Effect of Thermal Diffusion on Decarburization Kinetics. Metall Mater Trans B 45, 1974–1978 (2014). https://doi.org/10.1007/s11663-014-0211-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0211-z