Abstract
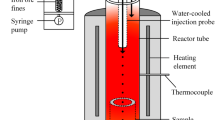
A novel flash ironmaking process based on hydrogen-containing reduction gases is under development at the University of Utah. The goal of this work was to study the possibility of the re-oxidation of iron particles in a H2-H2O gas mixture in the lower part of the flash reactor from the kinetic point of view. The last stage of hydrogen reduction of iron oxide, i.e., the reduction of wustite, is limited by equilibrium. As the reaction mixture cools down, the re-oxidation of iron could take place because of the decreasing equilibrium constant and the high reactivity of the freshly reduced fine iron particles. The effects of temperature and H2O partial pressure on the re-oxidation rate were examined in the temperature range of 823 K to 973 K (550 °C to 700 °C) and H2O contents of 40 to 100 pct. The nucleation and growth kinetics model was shown to best describe the re-oxidation kinetics. The partial pressure dependence with respect to water vapor was determined to be of first order, and the activation energy of re-oxidation reaction was 146 kJ/mol. A complete rate equation that adequately represents the experimental data was developed.















Similar content being viewed by others
References
H.Y. Sohn, M.E. Choi, Y. Zhang, and J.E. Ramos: Iron Steel Technol. (AIST Trans.), 2009, vol. 6 (6), pp. 158–65.
M.E. Choi: Ph.D. Dissertation, University of Utah, Salt Lake City, Utah, May 2010.
H. Y. Sohn: Steel Times Int., 2007, vol. 31 (4), pp. 68–72.
Outokumpu HSC Chemistry 5.1 for Windows—Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database, Outokumpu Research Oy, Pori, Finland.
P. Kaushik and R. J. Fruehan: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 715–25.
A. Bandopadhyay, A. Ganguly, K. K. Prasad, S. B. Sarkar, and H. S. Ray: Iron Steel Inst. Jpn. Int., 1989, vol. 29 (9), pp. 753–60.
A. A. El-Geassy, F. O. El-Kashif, M. I. Nasr, and A. A. Omar: Iron Steel Inst. Jpn. Int., 1994, vol. 34 (7), pp. 541–47.
W.R. Schütze: HBI—Hot Briquetting of Direct Reduced Iron Technology and Status of Industrial Application, Maschinenfabrik Köppern GmbH & Co. KG, Hattingen, Germany, 1999.
M.E. Choi and H.Y. Sohn: Ironmak. Steelmak., 2010, vol. 37 (2), pp. 81–88.
H. Wang and H.Y. Sohn: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 133–45.
International Organization for Standardization: Direct Reduced Iron—Determination of Metallic Iron-Bromine-Methanol Titrimetric Method, ISO 5416, 3rd ed., Geneva, Switzerland, 2006.
J. Szekely, J.W. Evans, and H.Y. Sohn: Gas–Solid Reactions, Academic Press, New York, 1976, pp. 73–81, 131.
H. Wang: Ph.D. Dissertation, University of Utah, Salt Lake City, Utah, May 2011.
S. Seetharaman and H.Y. Sohn: Fundamentals of Metallurgy, S. Seetharaman, ed., Woodhead Publishing Limited, Cambridge, U.K., 2005, pp. 299–310.
W. E. Boggs, R. H. Kachik and G. E. Pellissier: J. Electrochem. Soc., 1965, vol. 112 (6), pp. 539–46.
N.J. Themelis and W.H. Gauvin: Trans. Am. Inst. Min. Metall. Petrol. Eng., 1963, vol. 227, pp. 290–300.
S. K. El-Rahaiby and Y. K. Rao: Metall. Trans. B, 1979, vol. 10B, pp. 257–69.
P. C. Hayes: Metall. Trans. B, 1979, vol. 10B, pp. 211–17.
M. E. Choi, H. Y. Sohn, Y. M. Z. Ahmed, F. M. Mohamed, G. Han, and M. E. H. Shalabi: Chem. Eng. Technol., 2007, vol. 30 (5), pp. 628–34.
M. Avrami: J. Chem. Phys., 1939, vol. 7 (12), pp. 1103–12.
M. Avrami: J. Chem. Phys., 1940, vol. 8 (2), pp. 212–24.
M. Avrami: J. Chem. Phys., 1941, vol. 9 (2), pp. 177–84.
IUPAC: Compendium of Chemical Terminology, 2nd ed., Compiled by A.D. McNaught and A. Wilkinson, Blackwell Scientific Publications, Oxford, 1997, p. 85.
W. A. Johnson and R. F. Mehl: TMS-AIME, 1939, vol. 135, pp. 416–58.
P.W.M. Jacobs and F.C. Tompkins: Chemistry of the Solid State, W.E. Garner, ed., Butterworths, London, 1955, pp. 184–212.
E. T. Turkdogan, W. M. McKenwan, and L. Zwell: J. Phys. Chem., 1965, vol. 69 (1), pp. 327–34.
H. Yin, W. Y. D. Yuen and D. J. Young: Materials and Corrosion, 2012, vol. 63 (10), pp. 869–77.
W. W. Smeltzer: Acta Metall., 1960, vol. 8 (6), pp. 377–83.
H. Pfeiffer and C. Laubmeyer: Z. Elektrochem., 1955, vol. 59, pp. 579–83.
E.N. Fuller, P.D. Schettler, and J.C. Giddings: Ind. Eng. Chem., 1966, vol. 58 (5), pp. 18–27.
Acknowledgments
Thanks are due to Dr. Moo Eob Choi for helpful technical discussions. Gratitude is also extended to Jingzhu “April” Li for her help with the use of SEM. We acknowledge the financial support from the American Iron and Steel Institute (AISI) through a research service agreement with the University of Utah under AISI’s CO2 Breakthrough Program. This material also contains the results of work supported in part by the U.S. Department of Energy under Award Number DE-EE0005751.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted: February 24, 2013.
Appendix
Appendix
Evaluation of the Effect of Inter-particle Diffusion
Whether inter-particle diffusion has a significant effect on the overall rate of a gas–solid can be assessed by comparing the diffusion-controlled rate with the experimentally observed rate. In the reaction investigated in this work, water vapor diffuses through a porous layer of iron particles, as illustrated in Figure A1, and undergoes the following reaction:
The relationship between conversion and time under inter-particle diffusion control in a flat geometry is given by[12]
where t is the reaction time, X is the fractional conversion of the solid, K is the equilibrium constant of the reaction of interest, b is the stoichiometry coefficient of reaction (=1 for the reaction in question), \( F_{\text{p}} = 1 \), \( D_{\text{e}} \) is the effective gas phase diffusion coefficient, \( \in \) is the voidage of the particle bed, \( \left( {1 - \in } \right)\rho_{\text{s}} \) is the number of moles of solid reactant per unit volume of the bed, \( V_{\text{p}} \) and \( A_{\text{p}} \) are, respectively, the volume and surface area of the particle layer, and \( C_{{{\text{H}}_{2} {\text{O}}_{\text{b}} }} \) and \( C_{{{\text{H}}_{{2{\text{b}}}} }} \) are, respectively, the bulk molar concentration of water vapor and hydrogen. The time needed to reach complete conversion is
where \( L_{\text{p}} \) is the thickness of the particle layer. The equilibrium constant of Reaction [A1] at 873 K (600 °C) is
Molar density of iron particles is
The voidage of an iron particle bed of 100 mg with area of 9 cm2 and thickness of 0.5 mm is
and
Consider in the gas mixture, \( p_{{{\text{H}}_{2} {\text{O}}}} /p_{{{\text{H}}_{2} }} = 80\;{\text{pct}}/20\;{\text{pct}} \), (atmospheric pressure = 0.85 atm),
According to the Fuller–Schettler–Giddings[30] method, the gas phase diffusion coefficient can be obtained from the following relationship:
where T = 873 K (600 °C), total pressure P = 0.85 atm, molecular weights \( M_{{{\text{H}}_{2} {\text{O}}}} \) = 18 g/mol, \( M_{{{\text{H}}_{2} }} \) = 2 g/mol, \( \nu_{{{\text{H}}_{2} }} = 7.07 \), and \( \nu_{{{\text{H}}_{2} }} = 12.7 \).
Substitute all known variables to get
Therefore,
According to the above equation, if the thickness of a sample layer is 0.05 cm, the time required for the reaction to reach completion is 0.13 seconds. However, the observed rate was much slower than this calculated rate. Thus, it can be concluded that the reaction rate was not affected by the inter-particle diffusion.
Rights and permissions
About this article
Cite this article
Yuan, Z., Sohn, H.Y. & Olivas-Martinez, M. Re-oxidation Kinetics of Flash-Reduced Iron Particles in H2-H2O(g) Atmosphere Relevant to a Novel Flash Ironmaking Process. Metall Mater Trans B 44, 1520–1530 (2013). https://doi.org/10.1007/s11663-013-9910-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9910-0