Abstract
The liquidus surface projection and isothermal sections at 1100 and 1000 °C of the Co–Nb–Zr system were constructed by analyzing the solidification morphology of as-cast alloys and annealed microstructure of the equilibrated alloys and diffusion couples. Six primary solidification areas were obtained in the liquidus surface projection, and four primary solidification areas were deduced from the binary phase diagram. Additionally, three three-phase regions at 1100 °C and five three-phase regions at 1000 °C were determined in the isothermal sections. Based on the experimental information, a thermodynamic description of the Co–Nb–Zr system was established using the CALPHAD (CALculation of PHAse Diagram) method. The thermodynamic models of intermetallic phases Co11Zr2, Co23Zr6, CoZr2, Co7Nb2, λ1, λ2 and λ3, were described as (Co)11(Nb,Zr)2, (Co)23(Nb,Zr)6, (Co,Zr)(Nb,Zr)2, Co7Nb2, (Co,Nb)2(Co,Nb), (Co,Nb,Zr)2(Co,Nb,Zr) and (Co,Nb)2(Co,Nb), respectively. CoZr3 and μ were modeled as (Co,Zr)(Co,Zr)Zr2 and (Co,Nb,Zr)1(Nb,Zr)4(Co,Nb,Zr)2(Co,Nb,Zr)6. Moreso, CoZr with the structure of B2 was described as an ordered phase of bcc_A2. Thus, a reasonable thermodynamic description of the Co–Nb–Zr system was obtained, and the experimental information was well reproduced.
Similar content being viewed by others
1 Introduction
Co-based alloys can be widely used in critical industrial materials such as magnetic materials and superalloys due to the addition of Nb, Ta, Mo, Ti, Zr, V, and other elements.[1,2,3,4,5] Additionally, Nb improves the stability of γ’ and increases the volume fraction of γ′(L12)-precipitates.[6] Moreover, Nb can precipitate granular MC-type carbide at grain boundaries, improving the alloy’s lasting strength.[7] However, acicular or flaky secondary carbides are precipitated at the grain boundaries by adding Nb, which decreases the alloy’s plasticity and durability.[8] Additionally, Zr segregates at grain boundaries to reduce defects, thereby improving the strength and ductility of as-cast alloys.[9] Zr can also form MC-type carbides in Co-based superalloys, effectively strengthening the alloy matrix.[10] Notably, the atomic radii of Zr and Nb are close, and Nb and Zr with the same bcc structure can form an infinite solid solution bcc(Nb, Zr). Thus, alloys containing Nb and Zr have excellent corrosion resistance.[11] Therefore, it is essential for the phase relationships of the Co–Nb–Zr system to understand the phase stability and transformation in Co-based alloys with the addition of Nb and Zr.
The current work mainly investigates the phase relationships at 1100 and 1000 °C and the liquidus surface projection. The CALPHAD (CALculation of PHAse Diagram) method[12] is one of the efficient ways of material design. A thermodynamic description of the Co–Nb–Zr system by CALPHAD was performed, and the experimental information was reproduced with the obtained thermodynamic parameters. Importantly, the reasonable thermodynamic description of the Co–Nb–Zr system is integral to constructing a multicomponent thermodynamic database of Co-based superalloys.
2 Literature Information
2.1 The Co–Nb System
In the Co–Nb system, there are five intermetallics Co7Nb2, Co3Nb (λ3), Co2Nb (λ2), Co16Nb9 (λ1), and Co7Nb6 (μ).[13] Stein et al.[14] studied the Co–Nb system from 800 to 1380 °C utilizing diffusion couples and equilibrium alloys, and found that the compositions of Nb in the Laves phases of λ3, λ2, and λ1 ranged from 24.5 to 25.5 at. pct, 26 to 35.3 at. pct, and 35.8 to 37.4 at. pct, respectively. Subsequently, several scholars conducted the thermodynamic optimization of the Co–Nb binary phase diagram.[15,16,17,18,19] Furthermore, the thermodynamic optimization of the Co–Nb system was re-evaluated while evaluating the ternary system Co–Nb–Ti by Wei et al.,[19] which was able to match the Co-based superalloy thermodynamic database, and adopted in this work. The calculated Co–Nb phase diagram is exhibited in Figure 1(a).
2.2 The Co–Zr System
The Co–Zr phase diagram was first studied by Pechin et al.,[20] and they identified five compounds Co11Zr2, Co23Zr6, Co2Zr (λ2), CoZr, and CoZr2. Bataleva et al.[21] investigated the Co–Zr system and found the existence of CoZr3 by using metallography, EPMA, and XRD methods. More recently, Liu et al.[22] found that the temperature of peritectoid reaction bcc(Zr) + CoZr2 ↔ CoZr3 was about 985 °C by using DTA (Differential Thermal Analysis). Subsequently, many researchers assessed the thermodynamic parameters of the Co–Zr system.[23,24,25,26,27] Moreso, Durga and Kumar[23] first adopted an order–disorder model to couple bcc_A2 and CoZr with B2 structure, thereby improving the thermodynamic parameters of the Co–Zr system. To make \(G_{\rm Co:Co}^{{\lambda_{2} }}\) in the Co–Zr system consistent with \(G_{\rm Co:Co}^{{\lambda_{2} }}\) in the Co-Nb system, the thermodynamic parameters of λ2 in Reference 27 were re-optimized in this study. The calculated Co–Zr phase diagram is shown in Figure 1(b).
2.3 The Nb–Zr System
Guillermet[28] obtained the thermodynamic parameters of the Nb–Zr system based on analytical and experimental information. Recently, the thermodynamic parameters of the Nb–Si–Zr ternary system were evaluated by Li et al.[29] and were used in this work. The calculated Nb–Zr phase diagram is displayed in Figure 1(c). Furthermore, the crystallographic information of each compound in the Co–Nb–Zr system is outlined in Table I.
3 Experimental Procedures
3.1 Equilibrated Alloy Preparation
High-purity cobalt, niobium, and zirconium (99.99 wt. pct) were used as the raw materials for the as-cast and annealed samples. Each specimen of about 3 g was melted by arc melting (MTI MSM20-7) under the protection of a high-purity argon atmosphere. Pure titanium was used as an oxygen absorber and should be remelted thrice before smelting, and then the samples were smelted at least six times. Under the atmosphere of high-purity argon as the protective gas, the as-cast alloys were encapsulated in quartz tubes. The annealing times of alloys b1–b9, alloys b10–b17 and alloys b18–b23 were 40, 80 and 2 days at 1100 °C, respectively. Additionally, the annealing times of the alloys c1–c8, alloys c9–c17 and alloys c18–c23 were 50, 90 and 3 days at 1000 °C, respectively. Furthermore, the specimens were quenched into ice water following heat treatment.
3.2 Diffusion Couple Preparation
Even if a long heat treatment time was used, achieving equilibrium is difficult when some refractory elements are contained. The diffusion couple method has been used as an efficient method to study phase equilibrium based on the principle of local equilibrium.[30] Therefore, to further verify the phase relationships of the Co–Nb–Zr system obtained with the equilibrium alloy at 1000 °C, the diffusion couple method was used. The as-cast Nb80Zr20 (at. pct) alloy obtained by smelting was cut into the blocks of 5 mm × 5 mm × 8 mm and annealed at 1200 °C for 108 hours. Subsequently, the contacting surfaces for Nb80Zr20 and Co were metallographically polished and tied together with Mo wire to form a block of diffusion couples. Moreso, the Nb80Zr20/Co diffusion couple was encapsulated in a quartz tube and then annealed at 1000 °C for 360 hours. Finally, the diffusion couple was quenched in ice water.
3.3 Sample Analysis
The heat-treated samples were subjected to metallographic analysis under a scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS) at an accelerating voltage of 20.0 kV to determine the microstructures and phase compositions. Additionally, an XRD instrument obtained the XRD patterns of the specimens. Notably, the diffraction pattern has a scan step size of 0.02 deg in the 2θ range from 20 to 90 deg. Furthermore, EPMA-1720H (Shimadzu, Japan) with a wave dispersive X-ray spectrometer (WDS) was used to measure the phase equilibria between fcc(Co), λ2, μ, CoZr, and bcc(Nb, Zr) in the Nb80Zr20/Co diffusion couple.
4 Results and Discussion
4.1 Liquidus Surface Projection
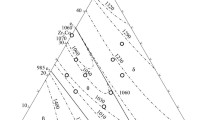
The primary solidification phase and the solidification paths of 48 alloys are displayed in Table II. The solidification paths of typical as-cast alloys are selected for discussion.
The primary solidification phase is fcc(Co) in alloys a1 to a3. Combined with the XRD pattern of alloy a1 in Figure 2(b), only two phases fcc(Co) and Co23Zr6 were determined. The black phase fcc(Co) as the primary solidification phase was observed in Figure 2(a). Moreso, fcc(Co) was first precipitated from the liquid phase, and the composition of the remaining liquid phase was at the univariate line of liq. → fcc(Co) + Co23Zr6. The microstructure and XRD patterns of alloy a3 are displayed in Figures 2(c) and (d), where the black phase fcc(Co) as the primary solidification phase and a eutectic structure fcc(Co) + λ2 were observed. Thus, there must be a monovariant line of liq. → fcc(Co) + λ2 between fcc(Co) and λ2 in the liquidus surface projection.λ2 as the primary solidification region was observed in alloys a4 to a21. However, the solidification structure is completely different due to the different solidification paths after λ2 precipitation. Alloys a4, a10, a11, a14, and a16 have the same solidification path. The microstructure and XRD patterns of alloy a4 are exhibited in Figures 3(a) and (b), where the phases λ2 and Co23Zr6 were determined. λ2 was first precipitated, and the liquid composition was at the univariate line of liq. + λ2 → Co23Zr6. Notably, alloys a5, a7, and a8 have identical solidification paths. Three phases, λ2, Co23Zr6, and fcc(Co), were determined according to the XRD pattern of alloy a5, as shown in Figure 3(d). The light phase λ2, the grey phase Co23Zr6, and a eutectic microstructure fcc(Co) + Co23Zr6 are shown in Figure 3(c). λ2 was first precipitated from the liquid phase, and the composition of the liquid was at the peritectic univariate line of liq. + λ2 → Co23Zr6. Then the remaining liquid phase moved to the monovariant line and transformed into fcc(Co) + Co23Zr6 microstructure. The solidification path can be surmised: liq. → λ2, liq. + λ2 → Co23Zr6, liq. → Co23Zr6 and liq. → fcc(Co) + Co23Zr6. Moreso, alloys a6, a9, a12, a13, and a15 have identical solidification paths. The microstructure and XRD patterns of alloy a9 are exhibited in Figures 3(e) and (f). λ2 was first precipitated from the liquid phase, and the composition of the liquid was at the univariate line of liq. → fcc(Co) + λ2. As shown in Figure 4(b), CoZr and λ2 were detected based on the XRD pattern of alloy a17. The dark phase λ2 and the light phase CoZr were observed as shown in Figure 4(a). A eutectic microstructure CoZr + λ2 was formed from the liquid phase after the precipitation of λ2. The microstructure and XRD patterns of alloy a19 are exhibited in Figures 4(c) and (d), where the black phase λ2 and the light phase μ were observed. λ2 was precipitated at the beginning, and then the remaining liquid phase moved to the monovariant line and transformed into λ2 + μ microstructure.
Alloys a22 to a26 have the same primary solidification phase as CoZr. The microstructure and XRD patterns of alloy a22 are exhibited in Figures 5(a) and (b), where CoZr and CoZr2 were observed. CoZr was first precipitated from the liquid phase, and the composition of the remaining liquid phase was at the univariate line of liq. → CoZr + CoZr2. Only three phases, bcc(Nb, Zr), CoZr, and CoZr2, were detected from the XRD patterns of alloy a25 in Figure 5(d). A eutectic microstructure bcc(Nb, Zr) + CoZr and a ternary eutectic microstructure bcc(Nb, Zr) + CoZr + CoZr2 were observed in Figure 5(c). The solidification path can be conjectured: liq. → CoZr, liq. → bcc(Nb, Zr) + CoZr and liq. → bcc(Nb, Zr) + CoZr + CoZr2.
The primary solidification region of μ was observed in alloys a27 to a39. The microstructure and XRD patterns of alloy a27 are displayed in Figures 5(e) and (f), where μ and CoZr were observed. μ was first precipitated from the liquid phase. Additionally, the liquid phase moved to the monovariant line of liq. → CoZr + μ, then the liquid composition was at the invariant transformation point liq. → CoZr + λ2 + μ. From the microstructure of alloy a35, single phase μ was observed as shown in Figure 6(a). It was further verified that only μ existed by the XRD pattern in Figure 6(b). So, the solidification path can be conjectured: liq. → μ. The black and white phases were μ and bcc(Nb, Zr) based on the analyses of the EDS and XRD patterns of alloy a36 in Figures 6(c) and (d). μ was first precipitated, then the liquid phase moved to the univariant line of liq. → bcc(Nb, Zr) + μ. The microstructure and XRD patterns of alloy a40 are exhibited in Figures 7(a) and (b). CoZr2 was solidified from the liquid phase, then the liquid composition was at the univariant line and transformed into bcc(Nb, Zr) + CoZr2 microstructure.
There were bcc(Nb, Zr), CoZr, and CoZr2 from the microstructure and XRD patterns of alloy a45, as shown in Figures 7(c) and (d). bcc(Nb, Zr) was first precipitated, and the liquid phase was at the monovariant line and transformed into bcc(Nb, Zr) + CoZr microstructure. However, the content of CoZr was minimal. Thus, the eutectic structure bcc(Nb, Zr) + CoZr loses the characterization of typical eutectic microstructure. Finally, the remaining liquid decomposed the ternary eutectic microstructure bcc(Nb, Zr) + CoZr + CoZr2. The microstructure and XRD patterns of alloy a46 are demonstrated in Figures 7(e) and (f), where bcc(Nb, Zr) and μ were observed. bcc(Nb, Zr) was first precipitated and the liquid composition was at the univariant line and transformed into bcc(Nb, Zr) + μ microstructure.
4.2 Isothermal Section at 1100 °C
Twenty-four annealed specimens were analyzed using SEM/EDS and XRD patterns to construct the phase relationships of 1100 °C. The constituent phases and compositions are outlined in Table III.
On the Co-rich side, the two-phase microstructures, fcc(Co) + λ2 in alloy b3 and Co23Zr6 + λ2 in alloy b8, and a three-phase microstructure fcc(Co) + Co23Zr6 + λ2 in alloy b5 were observed, as shown in Figure 8. The microstructure and XRD patterns of alloy b12 are displayed in Figures 9(a) and (b), where λ2 and μ were observed. A three-phase equilibrium CoZr + λ2 + μ was measured because of the microstructure and XRD patterns of alloy b14 in Figures 9(c) and (d). Additionally, the measured maximum solubilities of Nb in Co23Zr6 and CoZr were ~ 3.1 and ~ 11.9 at. pct, and the maximum solubility of Zr in μ was ~ 17.9 at. pct. The microstructures and XRD patterns of alloys b15 and b16 are demonstrated in Figures 9(e) and (f) and Figures 10(a) and (b), where two two-phase microstructures, CoZr + μ and bcc(Nb, Zr) + μ, were observed.
The phase related to liquid can be observed based on the phase constituents of alloys b20, b21, and b22. The phase equilibria, CoZr + liquid, bcc(Nb, Zr) + CoZr + liquid, and bcc(Nb, Zr) + liquid, were determined following the microstructures of Figures 10(c) and (d) and 11. Furthermore, a three-phase equilibrium bcc(Nb, Zr) + liquid + μ can be deduced according to the analyses of alloys b15 and b16.
According to the experimental results, the phase relationships of Co–Nb–Zr system were confirmed. Co2Nb and Co2Zr with the same C15 structure formed a continuous compound from Co2Nb to Co2Zr at 1100 °C. Three three-phase equilibria, fcc(Co) + Co23Zr6 + λ2, CoZr + λ2 + μ, and bcc(Nb, Zr) + CoZr + liquid, were obtained, and a three-phase region bcc(Nb, Zr) + CoZr + μ was deduced. Moreso, the maximum solubilities of Nb in Co23Zr6 and CoZr were ~ 3.1 at. pct and ~ 11.9 at. pct, and the maximum solubility of Zr in μ was ~ 17.9 at. pct.
4.3 Isothermal Section at 1000 °C
The experimental information of twenty-three samples annealed at 1000 °C is listed in Table IV. The analyses of several samples for three-phase equilibria are discussed as follows.
Three three-phase equilibria, fcc(Co) + Co23Zr6 + λ2 in alloy c5, CoZr + λ2 + μ in alloy c13, and bcc(Nb, Zr) + CoZr + μ in alloy c16, were determined based on the microstructures and XRD patterns as shown in Figure 12. The microstructures and XRD patterns of alloys c17 and c18 are presented in Figure 13, where the three-phase microstructures, bcc(Nb, Zr) + CoZr + CoZr2 and bcc(Nb, Zr) + CoZr2 + liquid, were determined. According to the experimental data above, the maximum solubilities of Nb in Co23Zr6, CoZr, and CoZr2 were ~ 2.8, ~ 11.3, and ~ 8.7 at. pct, and the maximum solubility of Zr in μ was ~ 17.5 at. pct, respectively.
Furthermore, typical diffusion layers of λ2 and μ were observed by the Nb80Zr20/Co diffusion couple annealed at 1000 °C as shown in Figure 14. Due to the presence of CoZr, a three-phase conjunction corresponding to bcc(Nb, Zr) + CoZr + μ was identified in Figure 14. The compositions of equilibrium phases of Nb80Zr20/Co diffusion couple obtained using EPMA at 1000 °C are shown in Table V, and tie lines are shown using the dashed lines with inverted triangles in Figure 15(b). Furthermore, the phase relationships and phase compositions obtained by the diffusion couple were almost identical to those of the equilibrated alloys, as shown in Figure 15(b).
5 Thermodynamic Modeling
5.1 Thermodynamic Model
The Gibbs energy functions for the unary phases of the pure elements Co, Nb, and Zr were taken from the SGTE database.[31]
The Gibbs energies of solution phases (liquid, bcc fcc, and hcp) in the Co–Nb–Zr system are expressed:
where xCo, xNb, and xZr indicate the molar fractions of the pure elements Co, Nb, and Zr, respectively. \({}^{{\text{E}}}G_{{\text{m}}}^{\phi }\) is the molar excess Gibbs energy, and its Redlich–Kister polynomial function can be described[32]:
in which \({}^{j}L_{{\text{Co,Nb}}}^{\phi }\), \({}^{j}L_{{\text{Co,Zr}}}^{\phi }\), and \({}^{j}L_{{\text{Nb,Zr}}}^{\phi }\) are the interaction parameters obtained from Co–Nb,[19] Co–Zr,[27] and Nb–Zr[29] systems. \(L_{{\text{Co,Nb,Zr}}}^{\phi }\) is the ternary interaction parameters and expressed as follows:
where \({}^{j}L_{{\text{Co,Nb,Zr}}}^{\phi } = a_{j} + b_{j} T\) (j = 0, 1, and 2). The factors of \(a_{j}\) and \(b_{j}\) were required to be optimized in this work.
CoZr with B2 structure is described as an ordered phase of bcc_A2 and modeled as (Co, Nb, Zr, Va)0.5(Co, Nb, Zr, Va)0.5(Va)3. The Gibbs energy function of CoZr can be constructed:
where Gmdis(xi) denotes the Gibbs energy of bcc_A2. \(G_{{\text{m}}}^{{{\text{ord}}}} (y_{i}^{\prime} ,y_{i}{\prime \prime} ) - G_{{\text{m}}}^{{{\text{ord}}}} (x_{i} )\) means the ordered contribution to Gibbs energy.
The intermediate compounds Co11Zr2, Co23Zr6, CoZr2, Co7Nb2, λ1, λ2 and λ3 are modeled as (Co)11(Nb,Zr)2, (Co)23(Nb,Zr)6, (Co,Zr)(Nb,Zr)2, Co7Nb2, (Co,Nb)2(Co,Nb), (Co,Nb,Zr)2(Co,Nb,Zr) and (Co,Nb)2(Co,Nb), respectively. CoZr3 and μ are described as (Co,Zr)(Co,Zr)Zr2 and (Co,Nb,Zr)1(Nb,Zr)4(Co,Nb,Zr)2(Co,Nb,Zr)6. Taking Laves phase λ2 as an example, the Gibbs energy function is expressed:
where \(y_{*}^{\prime }\) and \(y_{\ast}^{\prime \prime}\) are the fractions of sites of elements (* = Co, Nb, and Zr) on the first and second sublattices. \({}^{{\text{E}}}G_{{\text{m}}}^{{{\uplambda }_{{2}} }}\) is the excess Gibbs energy.
5.2 Assessment Procedure
The thermodynamic parameters of Co–Nb and Nb–Zr systems were taken from the literatures[19] and[29] in the Co–Nb–Zr system. To unify the thermodynamic parameters of phases with the same crystal structure in the Co-based superalloys thermodynamic database, the thermodynamic parameters of λ2 in the Co–Zr system[27] were slightly modified according to the corresponding thermodynamic parameters in the Co–Nb system.[29] Furthermore, the Co–Nb–Zr system was optimized using the PARROT module in Thermo-Calc software and the PanOptimizer module in Pandat software to comply with the experimental data of the current work.
5.3 Thermodynamic Modeling
Thermodynamic models and obtained thermodynamic parameters of each phase in the Co–Nb–Zr system are shown in Table VI. The calculated phase relationships at 1100 °C and 1000 °C in comparison with the experimental information are shown in Figures 15(a) and (b). Three three-phase equilibria, fcc(Co) + Co23Zr6 + λ2, CoZr + λ2 + μ and bcc(Nb,Zr) + CoZr + liquid, at the 1100 °C and five three-phase regions, fcc(Co) + Co23Zr6 + λ2, CoZr + λ2 + μ, bcc(Nb,Zr) + CoZr2 + liquid, bcc(Nb,Zr) + CoZr + CoZr2 and bcc(Nb,Zr) + CoZr + μ at 1000 °C were well reproduced in the calculated isothermal sections.
The comparison of the calculated primary solidification region with the experimental data of the Co–Nb–Zr system is shown in Figure 16(a), in which ten primary solidification regions, fcc(Co), bcc(Nb, Zr), Co11Zr2, Co23Zr6, CoZr, CoZr2, λ1, λ2, λ3, and μ in the liquidus surface projection were very well reproduced. The calculated liquidus surface projection with isotherms is exhibited in Figure 16(b), in which seven invariant reactions: liq. → CoZr + λ2 + μ, liq. → Co23Zr6 + fcc(Co) + λ2, liq. → Co11Zr2 + Co23Zr6 + fcc(Co), liq. → fcc(Co) + λ2 + λ3, liq. + μ → bcc(Nb, Zr) + CoZr, liq. + λ2 → λ1 + μ, liq. → bcc(Nb, Zr) + CoZr + CoZr2, and six maximum points, Max1, Max2, Max3, Max4, Max5 and Max6 on the monovariant lines were manifested.
The calculated solidification path by the Scheil model[33] was compared with those of alloys a35 and a45 in experiments to further verify the reliability of the calculated liquidus surface projection. It was found that only single-phase μ was observed from alloy a35, as shown in Figures 6(a) and (b). The solidification paths obtained by the Scheil model were as follows liq. → μ and liq. → bcc(Nb, Zr) + μ. When a eutectic microstructure bcc(Nb, Zr) + μ was precipitated, the contents of the remaining liquid phase was 5.5 at. pct using the Scheil model. Therefore, it was challenging to analyze EDS and XRD patterns. In addition, the calculated solidification path using the Lever model was liq. → μ. So, the calculated results were acceptable. There were only three phases, bcc(Nb, Zr), CoZr, and CoZr2, as shown in Figures 7(c) and (d), based on the analyses of the microstructure and XRD pattern of alloy a45. The solidification path of alloy a45 can be inferred: liq. → bcc(Nb,Zr), liq. → bcc(Nb,Zr) + CoZr and liq. → bcc(Nb, Zr) + CoZr + CoZr2, which was the same as the calculation of the solidification path by the Scheil model. Furthermore, the invariant reaction scheme related to liquid and the invariant reactions are exhibited in Figure 17 and Table VII.
6 Conclusions
The experimental liquidus surface projection of the Co–Nb–Zr system was constructed by analyses of solidification paths of as-cast alloys, where ten primary solidification regions and seven invariant reactions were obtained, respectively.
The experimental isothermal sections of the Co–Nb–Zr system at 1100 and 1000 °C were constructed. Three three-phase regions at 1100 °C and five three-phase regions at 1000 °C were also determined. Notably, Co2Nb and Co2Zr with the same C15 structure formed a continuous compound from Co2Nb to Co2Zr at 1100 and 1000 °C. The maximum solubilities of Nb in Co23Zr6 and CoZr were ~ 3.1 and ~ 11.9 at. pct at 1100 °C, and the maximum solubilities of Nb in Co23Zr6, CoZr and CoZr2 were ~ 2.8, ~ 11.3 and ~ 8.7 at. pct at 1000 °C. The maximum solubility of Zr in μ was ~ 17.9 at 1100 °C and ~ 17.5 at. pct at 1000 °C.
A thermodynamic description of the Co–Nb–Zr system was established using the CALPHAD method according to the experimental data of the current work. Furthermore, a set of reasonable thermodynamic parameters of the Co–Nb–Zr system was obtained, which can provide theoretical guidance for the composition design of Co-based superalloys.
References
J. Zhao, F. Ma, P. Liu, X. Liu, W. Li, and D. He: J. Mater. Eng. Perform., 2020, vol. 29, pp. 3736–44.
T. Nagase, Y. Iijima, A. Matsugaki, K. Ameyama, and T. Nakano: Mater. Sci. Eng. C, 2020, vol. 107, p. 110322.
Z. Han, X. Liu, S. Zhao, Y. Shao, J. Li, and K. Yao: Prog. Nat. Sci. Mater., 2015, vol. 25, pp. 365–69.
T. Omori, K. Oikawa, J. Sato, I. Ohnuma, U.R. Kattner, R. Kainuma, and K. Ishida: Intermetallics, 2013, vol. 32, pp. 274–83.
I. Povstugar, P.P. Choi, S. Neumeier, A. Bauer, C.H. Zenk, M. Göken, and D. Raabe: Acta Mater., 2014, vol. 78, pp. 78–85.
X. Liu, Y. Pan, Y. Chen, J. Han, S. Yang, J. Ruan, C. Wang, Y. Yang, and Y. Li: Metals, 2018, vol. 8, p. 563.
S.G. Huang, R.L. Liu, L. Li, O.V. Der Biset, and J. Vleugels: Int. J. Refract. Met. H Mater., 2008, vol. 26, pp. 389–95.
D.A. Sandoval, J.J. Roa, O. Ther, E. Tarrés, and L. Llanes: J. Alloys Compd., 2019, vol. 777, pp. 593–601.
X. Liu, C. Luo, M. Yang, S. Yang, J. Zhang, Y. Huang, J. Han, Y. Lu, and C. Wang: J. Phase Equilib. Diffus., 2020, vol. 41, pp. 3–14.
W. Gui, H. Zhang, M. Yang, T. Jin, X. Sun, and Q. Zheng: J. Alloys Compd., 2017, vol. 695, pp. 1271–78.
Y. Liu, L. Zhang, T. Pan, D. Yu, and Y. Ge: CALPHAD, 2008, vol. 32, pp. 455–61.
J. Ågren: Curr. Opin. Solid State Mater. Sci., 1996, vol. 1, pp. 355–60.
H. Okamoto: J. Phase. Equilib., 2000, vol. 21, p. 495.
F. Stein, D. Jiang, M. Palm, G. Sauthoff, D. Grüner, and G. Kreiner: Intermetallics, 2008, vol. 16, pp. 785–92.
K.C.H. Kumar, I. Ansara, P. Wollants, and L. Delaey: J. Alloys Compd., 1998, vol. 267, pp. 105–12.
C. He, F. Stein, and M. Palm: MRS Online Proceedings Library (OPL), 2008, p. 1128.
L. Zhou, C. Wang, Y. Yu, X. Liu, H. Chinen, T. Omori, R. Kainuma, and K. Ishida: J. Alloys Compd., 2011, vol. 509, pp. 1554–62.
C. He, F. Stein, and M. Palm: J. Alloys Compd., 2015, vol. 637, pp. 361–75.
D. Wei, X. Bai, C. Guo, R. Li, and Z. Du: J. Alloys Compd., 2022, vol. 924, 166516.
W.H. Pechin, D.E. Williams, and W.L. Larsen: Am. Soc. Met. Trans. Q., 1964, vol. 57, p. 744.
S.K. Bataleva, V.V. Kuprina, V.V. Burnasheva, V.Y. Markiv, and G.N. Ronami: Moscow Univ. Chem. Bull., 1970, vol. 25, pp. 33–36.
X. Liu, H. Zhang, C. Wang, and K. Ishida: J. Alloys Compd., 2009, vol. 482, pp. 99–105.
A. Durga and K.C. Kumar: CALPHAD, 2010, vol. 34, pp. 200–05.
A. Durga, K.C. Kumar, N. Moelans, and P. Wollants: J. Alloys Compd., 2010, vol. 334, pp. 173–81.
T. Kosorukova, P. Agraval, V. Ivanchenko, and M. Turchanin: in XI International Conference on Crystal Chemistry of Intermetallic Compounds, 2010, p. 52.
J.C. Gachon and J. Hertz: CALPHAD, 1983, vol. 7, pp. 1–2.
C. Zhou and H. Wang: J. Phase Equilib. Diffus., 2021, vol. 42, pp. 77–90.
A.F. Guillermet: Int. J. Mater. Res., 1991, vol. 82, pp. 478–87.
J. Li, Y. Guo, S. Yang, Z. Shi, C. Wang, and X. Liu: J. Alloys Compd., 2015, vol. 642, pp. 216–24.
A.A. Kodentsov, G.F. Bastin, and F.J.J. van Loo: J. Alloys Compd., 2001, vol. 320, pp. 207–17.
A.T. Dinsdale: SGTE Pure ELEMENTS (Unary) Database, version 4.5, 2006.
O. Redlich and A.T. Kister: Ind. Eng. Chem., 1948, vol. 40, pp. 345–48.
J. Sun, C. Ming, B. Yang, C. Guo, C. Li, and Z. Du: J. Alloys Compd., 2023, vol. 939, 168696.
Acknowledgments
this work was supported by Beijing Natural Science Foundation (Grant No. 2232077), National Natural Science Foundation of China (NSFC) (Grant No. 52271002) and the Cooperation Project of Jiangxi Provincial International Science and Technology (Grant No. 20212BDH81001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, J., Guo, C., Li, C. et al. Experimental Investigation and Thermodynamic Description of the Co–Nb–Zr System. Metall Mater Trans A 54, 3021–3044 (2023). https://doi.org/10.1007/s11661-023-07089-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-023-07089-7