Abstract
Retained austenite transformation was studied for a 5 wt pct Cr cold work tool steel tempered at 798 K and 873 K (525 °C and 600 °C) followed by cooling to room temperature. Tempering cycles with variations in holding times were conducted to observe the mechanisms involved. Phase transformations were studied with dilatometry, and the resulting microstructures were characterized with X-ray diffraction and scanning electron microscopy. Tempering treatments at 798 K (525 °C) resulted in retained austenite transformation to martensite on cooling. The martensite start (M s ) and martensite finish (M f ) temperatures increased with longer holding times at tempering temperature. At the same time, the lattice parameter of retained austenite decreased. Calculations from the M s temperatures and lattice parameters suggested that there was a decrease in carbon content of retained austenite as a result of precipitation of carbides prior to transformation. This was in agreement with the resulting microstructure and the contraction of the specimen during tempering, as observed by dilatometry. Tempering at 873 K (600 °C) resulted in precipitation of carbides in retained austenite followed by transformation to ferrite and carbides. This was further supported by the initial contraction and later expansion of the dilatometry specimen, the resulting microstructure, and the absence of any phase transformation on cooling from the tempering treatment. It was concluded that there are two mechanisms of retained austenite transformation occurring depending on tempering temperature and time. This was found useful in understanding the standard tempering treatment, and suggestions regarding alternative tempering treatments are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Industrial processes, such as rolling, cutting, forming, and punching, taking place at temperatures below 473 K (200 °C) are usually referred to as “cold work processes.” Steels that are used to make tools for these applications are, hence, called “cold work tool steels.”[1,2] These steels must be able of to attain properties, such as high hardness, high compressive strength, and good toughness, to be suitable for cold forming of advanced high-strength steels.
The fairly high alloying content of 5 wt pct Cr cold work tool steels results in low martensite start (M s ) and martensite finish (M f ) temperatures. Thus, the transformation of austenite to martensite does not go to completion when quenching to room temperature after austenitization. As a consequence, some amount of austenite is retained in the as-quenched microstructure, which otherwise consists of martensite (and sometimes B) and undissolved primary carbides. Before being put into use as a tool, the steel is then tempered to enhance its ductility and to precipitate alloy carbides. The tempering temperature influences both the stability of retained austenite and the precipitation of carbides in the tempered martensite; therefore, it has a large effect on the properties.[1,3,4,5]
Retained austenite transformation has been a subject of scientific interest for a long time, but characterization and understanding is still a challenge.[4,5,6,7,8] Literature suggests that the retained austenite can transform into ferrite and cementite,[6] martensite,[5] or B.[4] Ferrite and cementite form during isothermal treatment at the tempering temperature, while the latter transformations occur during cooling from the tempering temperature. It has been shown that the isothermal transformation into ferrite and carbide is a two-step process.[6,7,8,9] First, precipitation of cementite occurs from retained austenite, which is gradually followed by transformation into ferrite and cementite.[6] These observations have been reported for carbon, bainitic, and hot work tool steels.[10,11] Hence, the mechanism by which retained austenite transforms depends on steel composition as well as heat treatment procedure and is of great importance for the resulting mechanical properties.[12] Although the mechanisms are known, little information is available in the literature about the transformation of retained austenite during tempering of cold work tool steels.
The present investigation was designed to improve the understanding of retained austenite transformation during tempering and cooling of a 5 wt pct Cr cold work tool steel. With this purpose, tempering temperatures of 798 K and 873 K (525 °C and 600 °C) were selected and holding times were varied to understand the mechanisms involved during the transformation. The transformation behavior during heat treatments was studied with dilatometry, and the resulting microstructures were characterized with scanning electron microscopy (SEM) and X-ray diffraction (XRD). The results are used to understand the retained austenite transformation when the steel is tempered following the standard heat treatment.
2 Experimental
2.1 Material
An electroslag remelted ingot with the chemical composition of Uddeholm Caldie was produced. This was hot rolled into a round billet with a radius of 80 mm and then soft annealed. The chemical composition from the center of the steel billet is presented in Table I.
2.2 Heat Treatment
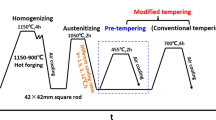
All specimens were heat treated in a push rod dilatometer (Dil 805 A/D, Bahr-Thermoanalyse). Cylindrical specimens (l × d = 10 × 4 mm) in soft-annealed condition were taken from the center of the steel billet. The specimens were heated with a high-frequency inductive coil in a vacuum chamber. Specimen temperatures were measured by a thermocouple, spot welded on the longitudinal surface of the specimen. The heat treatments consisted of hardening and tempering. All specimens were heated to 1323 K (1050 °C) with a rate of 3.5 K/s, held for 30 minutes, followed by cooling to room temperature. Helium gas was used as a quenching medium to achieve a cooling time of 300 seconds between 1073 K and 773 K (800 °C and 500 °C). Heating to the tempering temperature was done at a rate of 17 K/s. In the standard tempering treatment, the temperature was held for 2 hours before cooling to room temperature. This cycle was repeated two times (Figure 1).
However, understanding retained austenite transformation required tempering treatments with variations in both temperatures and holding times. Heat treatments conducted for the study are listed in Table II, where tempering at 798 K (525 °C) includes a range of holding times, the standard tempering consisting of a double tempering cycle at 798 K (525 °C) (Figure 1) and long-time tempering at 873 K (600 °C). The 798 K (525 °C) heat treatments with increasing holding times were conducted to investigate the stability of retained austenite. This was done by examining the correlation between the changes in the composition of retained austenite during tempering and its transformation to martensite on cooling. The 873 K (600 °C) treatment with long holding time was aimed at understanding retained austenite transformation during isothermal tempering.
2.3 Microstructural Characterization
Microscopy was carried out on dilatometry specimens by SEM (FEI Quanta 600F). Sample preparation involved grinding of the specimen with abrasive papers of mesh size 180, 300, 500, and 1200. The surfaces were polished first to 3 µm and then 1 µm with diamond suspension. The as-quenched microstructure was revealed by etching with 2 pct Nital for 1 minute, while tempered microstructures were etched first in Picral for 2 seconds and then in 2 pct Nital for 5 seconds. The microstructure constituents bainite, fresh martensite, tempered martensite, and retained austenite are referred to with the acronyms B, FM, TM, and RA, respectively, going forward.
XRD was conducted with Seifert 3003 equipment to measure the volume percent and the lattice parameter of RA. XRD was carried out with unfiltered Cr K α radiation at a voltage of 40 kV and a step size of 0.1 deg over a 2θ range of 50 to 165 deg. The software Rayflex 2.408 (GE & Inspection Technologies) was used to measure the relative intensities of the diffracted peaks of martensite (110) α , (200) α , and (211) α and the diffracted peaks of RA (111) γ , (200) γ , and (220) γ . Use of these numbers of peaks avoids possible bias due to crystallographic texture.[13] The curve fitting of the diffracted peaks was performed on the plot of intensity as a function of 2θ. The volume percent of RA was determined by comparing the integrated intensities of martensite and RA according to Cullity.[14] The uncertainty of measurements was estimated to ±2 vol pct. Retained austenite contents of less than 2 vol pct, therefore, cannot be measured reliably by XRD.
The lattice parameter (a γ ) was calculated for individual peaks of austenite, as described in Reference 15, and then plotted as a function of cos2 θ/sin2 θ. The final value of lattice parameter was obtained by extrapolating the linear fit against θ to 90 deg. The weighing of the data set is described elsewhere,[16] and the highest weight was given to the largest value of 2θ. The XRD measurements were repeated 3 times, and the standard deviation of the lattice parameter was calculated.
Dilatometry data were used to construct two types of plots: plots of length change (∆L) against specimen temperature (∆T) during cooling, where transformation start and finish temperatures were evaluated by calculating the derivative of length change as a function of specimen temperature. The start temperature for transformation was approximated as the initial deflection in the derivative after which the derivative was no longer constant.[17] The standard error of measuring start and finish temperatures was estimated as ±5 °C. Plots of relative length change against time during isothermal tempering were also constructed. The dilatometer has a resolution of 0.05 µm/0.05 °C (∆L/∆T).
Thermo-Calc, a software for thermodynamic calculation, was used to calculate the chemical composition and volume percent of all equilibrium phases. The calculation was performed for the composition of the investigated steel (provided in Table I) at 1323 K (1050 °C) and by using Uddeholms internal data base “Tooling 11.” The motivation was to predict the composition of the austenite after austenitization to permit a comparison with the carbon content of RA after tempering treatments.
3 Results
3.1 Thermodynamic Calculations
The thermodynamic calculations showed two phases in equilibrium at 1323 K (1050 °C), i.e., austenite and M7C3 carbide. The phase diagram of the investigated steel can be found elsewhere.[18] The equilibrium chemical composition and volume percentage of the phases are presented in Table III. It was predicted that austenitization would dissolve most of the alloying elements in austenite (compare Tables I and III). However, the remaining alloying content (i.e., 0.09 wt pct C, 0.38 wt pct Cr, and 0.1 wt pct V) was still tied up in undissolved primary carbides.
3.2 Microstructure
The microstructure of an as-quenched specimen is presented in Figure 2(a). The micrograph shows etched regions, unetched regions, and undissolved large primary carbides. Etched regions appear darker while the unetched regions appear lighter and have a blocky shape. The microstructures of specimens tempered at 798 K (525 °C) for holding times of 0.1, 0.5, 1, 2, or 6 hours had similar amounts of etched and unetched blocky regions, although they were smaller than for the as-quenched specimen. A representative microstructure (tempered for 2 hours) is shown in Figure 2(b). It consists of etched regions with smaller carbides (light phase), blocky regions, and undissolved large primary carbides. The microstructure after tempering at 798 K (525 °C) for 10 hours is shown in Figure 2(c). Similar to microstructures obtained for shorter aging times, there are etched regions with smaller carbides and blocky regions. However, the blocky regions are not as prominent as for shorter aging times, and a higher magnification micrograph (Figure 2(d)) reveals the presence of small carbides in the blocky regions.
SEM micrographs showing the microstructure of specimens in (a) as-quenched condition and tempered at 798 K (525 °C) for (b) 2 h and (c) 10 h containing etched and blocky unetched regions. The etched regions in tempered microstructures contained precipitation of carbides (light phase). After prolonged tempering, (c) the blocky regions were less prominent and (d) contained very small carbides as revealed at higher magnification
The microstructure of a specimen tempered twice at 798 K (525 °C) is presented in Figure 3. It is similar to the microstructure of the specimen tempered one time at 798 K (525 °C) (Figure 2(b)), i.e., showing carbides (light phase) in etched regions. However, a difference is the significant reduction in the amount of less etched blocky regions compared with the specimens tempered once.
The microstructure of specimens tempered at 873 K (600 °C) for 30 hours is shown in Figure 4(a). It shows undissolved primary carbides, regions with a dense precipitation of carbides, and regions that have a blockier appearance. The higher magnification micrograph in Figure 4(b) revealed that the blocky regions consist of carbides (gray) in a ferritic matrix.
3.2.1 XRD measurements
Results from XRD measurements are shown in Table IV. The as-quenched specimen contained significant amounts of RA, while the content successively decreased with increasing holding times at 798 K (525 °C). A measurable amount of RA was neither found in the specimens tempered for longer times (6 or 10 hours) at 798 K (525 °C) or at 873 K (600 °C) (30 hours) nor in the specimen tempered 2 × 2 hours at 798 K (525 °C).
The lattice parameter of the RA after tempering for 0.1 hours increased significantly compared to the lattice parameter of RA in as-quenched condition. However, the lattice parameters were reduced for specimens that were tempered at 798 K (525 °C) for holding times of 0.5, 1, and 2 hours (Table IV) compared with the lattice parameter of RA found in the specimen tempered for 0.1 hours.
3.3 Dilatometry
3.3.1 Hardening
Dilatometry investigations were performed on specimens that were quenched from the austenitization temperature. An example is presented in Figure 5, showing the length change and the derivative of the length change as a function of specimen temperature. The B at 473 K (200 °C) and M s temperature at 423 K (150 °C) are indicated on the derivative curve.
3.3.2 Tempering
3.3.2.1 Single tempering treatments
A plot of relative length change against holding time when tempering at 798 K (525 °C) for 10 hours is presented in Figure 6. It shows a continuous decrease in length of the specimen with a total contraction of 0.01 pct. All specimens tempered at 798 K (525 °C) closely followed the same curve of relative length change with time. When tempering at 873 K (600 °C) for 30 hours, contraction occurred for approximately 2 hours with a contraction of 0.01 pct, whereas from 2 to 30 hours, the length of the specimen increased with a total expansion of 0.1 pct, as presented in Figure 6.
Dilatometry plot of relative length change against holding time during tempering. The length decreased continuously with a total contraction of 0.01 pct when tempering at 798 K (525 °C) for 10 h (blue curve). The length decreased for the first 2 h with a contraction of 0.01 pct, while from 2 to 30 hours, a total expansion of 0.1 pct was observed on tempering at 873 K (600 °C) (red curve) (Color figure online)
3.3.2.2 Cooling after single tempering treatments
Dilatometry curves showing length change against specimen temperature on cooling after tempering at 798 K (525 °C) from 0.1 to 10 hours are presented in Figure 7. It also presents the length change of the specimen on cooling after tempering at 873 K (600 °C) for 30 hours. No bainitic transformation was observed, but start and finish temperatures for martensite transformation were identified and found to increase with longer time at holding temperature.
Dilatometry plot of relative length change as a function of specimen temperature during cooling to room temperature after tempering at 798 K (525 °C) for 0.1 (T 0.1), 0.5 (T 0.5), 1 (T 1), 2 (T 2), 6 (T 6), and 10 h (T 10) and specimen tempered at 873 K (600 °C) for 30 h (T 30). M s temperature increased with longer time at holding temperature. Notice that no martensitic transformation was observed for the specimen tempered at 798 K (525 °C) for 0.1 h or for the specimen tempered at 873 K (600 °C) for 30 h
A summary of M s and M f temperatures is presented in Table V. A clear trend can be seen that a longer holding time at 798 K (525 °C) increased the M s temperature for holding times of 0.5, 1, 2, 6, and 10 hours. After tempering for 6 or 10 hours, also M f temperatures can be identified. However, M s temperatures were not observed when tempering for 0.1 hours at 798 K (525 °C) or when tempering for 30 hours at 873 K (600 °C).
3.3.2.3 Double tempering treatment
A dilatometry plot showing relative length change against holding time for the second tempering at 798 K (525 °C) for 2 hours is presented in Figure 8(a), showing a total contraction of 0.002 pct. The M s temperature when cooling to ambient temperature after the second tempering was 180 °C, as shown in the dilatometry plot of Figure 8(b).
4 Discussion
Previous studies on the same steel showed that RA transformation occurred only when tempering at temperatures above 723 K (450 °C).[18] Thus, tempering temperatures of 798 K and 873 K (525 °C and 600 °C) were selected to study the transformation of RA. A summary of important observations is provided in Table VI as a starting point for a more detailed discussion. Note that in Table VI a RA content of less than 2 pct is considered to be zero.
4.1 Microstructure
The martensitic and bainitic transformations (Figure 5) resulted in an as-quenched microstructure with etched and unetched regions (Figure 2(a), Table VI). When comparing the as-quenched microstructure to the tempered microstructures (Figures 2(b) and (c)), it could be seen that carbides precipitated in the etched regions for all tempering treatments, and these, therefore, were interpreted as consisting of B or martensite. However, it is not possible, with certainty, to differentiate between B and martensite.
The unetched regions in the as-quenched microstructure had a blocky appearance, and carbides did not precipitate in these for short aging times. Thus, the blocky regions in the as-quenched microstructure were concluded to be mainly RA. However, a rough estimation showed that the amount of the blocky regions in the as-quenched microstructure was higher than the volume percent of RA (i.e., 24 pct). It is known that martensite may etch differently depending on the crystallographic orientations.[19] Thus, it is probable that some martensite was not etched and contributed to the as-quenched blocky regions.
The similar amounts of blocky regions in tempered microstructures (i.e., 0.1, 0.5, 1, 2, 6, or 10 hours) were not consistent with the content of RA (Tables IV and VI), proposing that the blocky regions in all tempered microstructures were not only RA. Rather, they were composed of a mix of RA and FM formed on cooling after tempering. An estimation of the amount of FM for tempered specimens is calculated as the difference in RA content (Table IV) and compared to the as-quenched microstructure in Table VII.
For the standard double tempering treatment, the 19 pct of FM formed during cooling from the first tempering was tempered during the second tempering and cooling from the second tempering resulted in transformation of the remaining 5 pct of RA to FM. These estimates were in line with the observed microstructures of specimens tempered twice (Figure 3); i.e., the number of blocky regions was significantly reduced.
4.2 Mechanisms of RA Transformation
Retained austenite transformation to martensite was observed on cooling after tempering for a range of holding times at 798 K (525 °C), while RA transformed to ferrite and carbides during tempering for a long time at 873 K (600 °C). The mechanisms involved are discussed in Sections I through III.
4.2.1 Transformation to martensite
A contraction of specimens was observed by dilatometry while holding at 798 K (525 °C). Literature suggests that the contraction is due to precipitation of carbides.[6,10] It is known that tempering of tool steels at 798 K (525 °C) can precipitate cementite[3] and nanosize MC and M2C tempering carbides[20,21] in the martensite. On the other hand, B contains only a slight excess of carbon in solution; thus, a large contribution from B is not expected during tempering.[4] Microscopy did indeed show precipitation in TM and B, but precipitation in blocky regions was only seen after longer holding times. This is a reflection of a large driving force for precipitation of carbides and a more rapid diffusion of carbon in martensite than in RA.[22] As suggested by the changes in the RA lattice parameter (Table IV), it is likely that precipitation starts already at shorter aging times but on a scale too fine to be observed by SEM. Further, M s and M f temperatures increased with longer holding time at 798 K (525°C) (Figure 9), suggesting that the RA changed to a less stable condition. At the same time, the lattice parameter of RA decreased, which is likely to be due to changes in its carbon content. Thus, an attempt was made to estimate the carbon content of RA to better understand the carbide precipitation and destabilization of the austenite.
The amount of carbon in RA was calculated from Eq. [1] for M s temperature. This equation was selected, as it is valid for steels containing less than 0.6 wt pct C, 4.9 wt pct Mn, 5.0 wt pct Cr, 5.0 wt pct Ni, and 5.4 wt pct Mo.[23] Although this composition deviates significantly from the steel in the present work, it was still assumed that this equation could be used.
The equilibrium content (weight percent) of substitutional elements in austenite at the austenitization temperature (Table III) and the measured M s temperatures (Table V) were used in Eq. [1] to calculate the wt pct C in RA. It was assumed that the substitutional alloying of RA does not change significantly during tempering.
The carbon content was also calculated from the lattice parameter of RA. Several equations can be found in the literature relating the effects of carbon content and substitutional alloying element to the lattice parameter of RA at room temperature. The equation used was selected on the basis of similarity in alloying elements to the studied steel and is given as[24,25,26]
where a γ is the lattice parameter of RA (Å) and C γ the carbon content (wt pct) of austenite. The compositions of substitutional alloying elements (weight percent) were taken from Table III. The carbon contents of RA calculated with Thermo-Calc and from Eqs. [1] and [2] were plotted against holding time at 798 K (525 °C) and are presented in Figure 10.
The trends are similar for the austenite carbon content curves calculated from M s temperatures or from lattice parameters showing an initial increase (discussed later) followed by a decrease for longer aging times. It can also be noticed that the carbon content of austenite predicted by Thermo-Calc simulation (0.61 pct, Table III) is close to the as-quenched value calculated from Eq. [2] (0.58 pct, Figure 10). The main difference is the higher carbon contents predicted by the M s formula compared to the other approaches. However, the trends rather than absolute values are important for understanding mechanisms. Hence, it was concluded that the increase in M s temperature (destabilization of RA) and the decrease in lattice parameter for longer aging times are associated with depletion of carbon in RA due to precipitation of carbides (either cementite or alloy carbides).
The decrease in M s temperatures (Figure 5 and Table V) and the accompanying increase of lattice parameter (Table IV) for the shortest aging times confuse the interpretation of results, as these observations point toward a higher content of carbon dissolved in the austenite retained after short tempering treatments. Similar observations can be found in the literature[22,27,28,29] and are explained with carbon diffusion (partitioning) from martensite to austenite or relaxation of stresses in the microstructure. It is not clear what the dominating process is or if other unidentified mechanisms are also involved. For the purpose of this article, the important observation is that M s continuously increases and lattice parameters continuously decrease except for the shortest aging times. This points, as discussed previously, toward destabilization of the RA due to precipitation of carbides.
4.2.2 Transformation to ferrite and carbides
Dilatometry showed a contraction during the first 2 hours of tempering at 873 K (600 °C) followed by expansion (Figure 6). The initial contraction is assumed to be due to precipitation of carbides[6,10] in B, martensite, and RA. Expansion, on the other hand, was attributed to transformation of the RA to ferrite in line with results from the literature.[6,10] Furthermore, the interpretation that ferrite formation causes the expansion was supported by the appearance of the microstructure (Figure 4), XRD results (Table IV), and the fact that dilatometry did not reveal any transformation on cooling from tempering. Taken together, this suggests that all the RA was decomposed into ferrite and carbides during holding at the tempering temperature.
4.2.3 Summary of mechanisms
It was concluded that two different mechanisms of RA transformation occurred, depending on the choice of tempering temperature and holding time. Martensite formed on cooling from 798 K (525 °C) for aging times up to 10 hours, while decomposition of RA to ferrite and carbides occurred during tempering at 873 K (600 °C). Both mechanisms required initial carbide precipitation from RA, resulting in lowering of the carbon content and destabilization of the RA. As a consequence, in the former process, the M s temperature increased above ambient and transformation to martensite became possible on cooling. However, the latter process occurred in two steps: RA first precipitated carbides gradually followed by the formation of ferrite and carbides as the austenite became depleted in carbon. The similar contraction during tempering for 10 hours at 798 K (525 °C) and the first 2 hours at 873 K (600 °C) (Figure 6) suggests that transformation to ferrite and carbides is likely to occur also at 798 K (525 °C) for longer holding times.
4.3 Standard Tempering Treatment 798 K (525 °C), 2 × 2 Hours)
Knowledge about the mechanisms of RA transformation was useful for a deeper understanding of the standard double tempering treatment of the steel. It is clear that RA transformed to martensite on cooling after the first and second tempering and that RA transformation to ferrite and carbides did not occur. It was concluded that fine scale carbide precipitation resulted in lowering of the content of carbon in RA and provided the necessary condition for transformation to martensite on cooling. Large amounts of martensite, B (i.e., 76 pct), and RA (i.e., 24 pct) precipitated carbides during the first tempering and resulted in a larger decrease in strain (0.007 pct), while 19 pct of martensite and 5 pct of RA precipitated carbides during the second tempering, resulting in a smaller strain (0.002 pct).
Tempering treatments for cold work tool steels serve two main purposes, depending on the application area of the tool: to avoid RA and to achieve better mechanical properties of the tools. Regarding the first purpose, alternative heat treatment parameters can be suggested based on this study. A longer holding time during the first tempering may increase the chance of transforming all RA during the first cooling, as seen when tempering at 798 K (525 °C) for 6 or 10 hours (Table IV). Therefore, a second tempering with a short holding time can be used to temper the freshly formed martensite. It was also seen that FM remained in the microstructure after the standard double tempering treatment. Another approach, therefore, would be to apply triple tempering treatments with shorter holding times to achieve a fully tempered microstructure. Furthermore, a single tempering treatment at 873 K (600 °C) with longer holding time would transform the RA to ferrite and carbides, although is not likely to produce the desired properties. Naturally, effects on mechanical properties would have to be explored before implementing alternative heat treatment procedures.
5 Conclusions
Retained austenite transformations were studied for a 5 wt pct Cr cold work tool steel when tempering at 798 K or 873 K (525 °C or 600 °C) with variation in holding times. The following was concluded.
-
1.
Retained austenite transformation to martensite occurred on cooling to ambient temperature after tempering at 798 K (525 °C).
-
2.
M s and M f temperatures increased, while the lattice parameter of RA decreased with longer holding times at 798 K (525 °C).
-
3.
Carbon content of RA decreased as a result of precipitation of carbides during holding at the tempering temperature of 798 K (525 °C), for 0.5 hours or more, resulting in destabilization of the austenite and an increase of the M s temperature on subsequent cooling to room temperature.
-
4.
Long-time tempering at 873 K (600 °C) resulted in transformation to ferrite and carbides during the tempering treatment.
References
G. Roberts, G. Kraus, and R. Kennedy: Tool Steels, 5th ed., ASM International, Materials Park, Metals Park, 2000, 79–103.
Uddeholm Steel for Cold Work Tooling, 9th ed., 2014, http://www.uddeholm.com/files/AB_cold_work_eng.pdf, accessed 11 02 2016.
G.R. Speich and W.C. Leslie: Metall. Trans.,1972, vol. 3, pp. 1043–54.
H.K.D.H. Bhadeshia: Bainite in Steels, 2nd ed., IOM Commercial Ltd., London, 2001, pp. 307–10.
H.K.D.H. Bhadeshia and R.W.K. Honeycombe: Steels: Microstructure and Properties, 3rd ed., Elsevier Ltd., Oxford, United Kingdom, 2006, pp. 183–207.
A.S. Podder and H.K.D.H. Bhadeshia: Mater. Sci. Eng. A, 2010, vol. 527, pp. 2121–28.
M.J. Van Genderen, M. Isac, A. Böttger, and E.J. Mittemeijer: Metall. Mater. Trans. A, 1997, vol. 28A, pp. 545–61.
S. Primig and H. Leitner: Thermochim. Acta, 2011, vol. 526, pp. 111–17.
P.V. Morra, A.J. Bötter, and E.J. Mittemeijer: J. Therm. Anal. Calorim., 2001, vol. 64, pp. 905–14.
C. Lerchbacher, S. Zinner, and H. Leitner: Mater. Sci. Eng. A, 2013, vol. 564, pp. 163–68.
C. Lerchbacher, S. Zinner, and H. Leitner: Micron, 2012, vol. 43, pp. 818–26.
M. Sarikaya, A.K. Jhingan, and G. Thomas: Metall. Trans. A, 1983, vol. 14A, pp. 1121–33.
M.J. Dickson: J. Appl. Crystallogr., 1969, vol. 2, pp. 176–80.
B.D. Cullity: Elements of X-ray Diffraction, 2nd ed., Addison-Wesley, Reading, 1978, p. 411.
C. Suryanarayana and M.G. Norton: X-Ray Diffraction, A Practical Approach, Plenum Publishing Corp., New York, NY, 1998, p. 101.
C. Garcia-Mateo, M. Preet, F.G. Caballero, and H.K.D.H. Bhadeshia: Mater. Sci. Technol., 2004, vol. 20, pp. 814–18.
Standard practice for quantitative measurement and reporting of hypoeutectoid carbon and low-alloy steel phase transformations. ASTM International, 2010, https://compass.astm.org Accessed 08 July 2016.
M.A. Rehan, A. Medvedeva, B. Högman, L.E. Svensson, and L. Karlsson: Steel Res. Int., 2016, vol. 87, pp. 1609–18.
G.F. Van der Voort: Metallography Principles and Practice, ASM International, Materials Park, OH, 1999, pp. 166–67.
J. Akre, F. Danoix, H. Leitner, and P. Auger: Ultramicroscopy, 2009, vol. 109, pp. 518–23.
K. Stiller, L.E. Svensson, P.R. Howell, W. Rong, H.O. Andren, and G.L. Dunlop: Acta Metall., 1984, vol. 32, pp. 1457–67.
M. WieBner, S. Kleber, and A. Kulmburg: Particle Particle Syst. Charact., 2005, vol. 22, pp. 407–17.
K.W. Andrews: J. Iron Steel Inst., 1965, vol. 203, pp. 721–27.
D.J. Dyson and B. Holmes: J. Iron Steel Inst., 1970, vol. 208, pp. 469–74.
N. Ridley, H. Stuart, and L. Zwell: Trans. TMS-AIME, 1969, vol. 245, pp. 1834–36.
F.G. Caballero, C. Capdevila, and C.G.D Andres: ISIJ Int., 2001, vol. 41, pp. 1093–1102.
P.M. Yushkevich: Metalloved. Obrab. Met., 1960, vol. 2, pp. 14–20.
L.I. Kogan and R.I. Entin: Fiz. Met. Metalloved., 1968, vol. 25, pp. 383–84.
V.A. Landa: Metalloved. Term. Obrab. Met., 1963, vol. 3, pp. 2–6.
Acknowledgments
The authors gratefully acknowledge Uddeholms AB and the Swedish Knowledge Foundation for funding this project, which is part of the research school “Simulation and Control of Material Affecting Processes.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 21, 2016.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rehan, M.A., Medvedeva, A., Svensson, LE. et al. Retained Austenite Transformation during Heat Treatment of a 5 Wt Pct Cr Cold Work Tool Steel. Metall Mater Trans A 48, 5233–5243 (2017). https://doi.org/10.1007/s11661-017-4232-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-017-4232-5