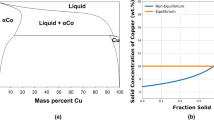
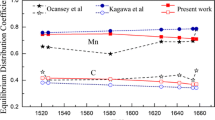
Abstract
In this article, a profile-fitting methodology was developed to measure the partition coefficients of solute elements during the solidification of Ni-base alloys. Better agreement with the theoretically calculated values is expected if the accuracy of the composition and the homogeneity of the model alloys are enhanced. Regular differential thermal analysis (DTA) measurements were consistently higher than the theoretical transition temperatures, and the differences were smaller when compared to the predictions performed with the thermodynamical database developed by Du et al. The better agreement between the experimental results and the theoretical predictions made with the newly developed database suggests that improvements in the accuracy of the theoretical predictions can still be obtained and are necessary for accurate freckling prediction. Quenching modified DTA (MDTA) experiments were proven to be appropriate for directly measuring the average partition coefficients of the solute elements. Regarding the cooling rate of the first stage of the quenching experiments, it was assumed successfully that the cooling rate prior to the quenching step of 0.083 Ks−1 was sufficiently slow to permit easy quenching, while being fast enough for the primary solidification reaction to depart from the equilibrium model and being closer to the Scheil model of segregation. The minimization of the error function defined from the Scheil equation was found to be an appropriate method for describing the segregation profiles of the quenched samples and permitted good estimations of the partition coefficients of the solute elements. The reliability of the methodology was found to be satisfactory given that the magnitudes calculated for the partition coefficients of the solutes in the multicomponent alloy 718 were found to be very close to the values reported in the literature.















Similar content being viewed by others
Notes
INCONEL and WASPALOY are trademarks of Special Metals Corporation, Huntington, WV.
Matlab is a registered trademark of The MathWorks, Inc., Natick, MA.
References
P.W. Schilke and R.C. Schwant: in Superalloys 718, 625, 706 and Various Derivatives, E.A. Loria, ed., TMS, Warrendale, PA, 2001, pp. 25–34.
R.C. Schwant, S.V. Thamboo, A.F. Anderson, C.B. Adasczik, B.J. Bond, and J.F. Uginet: in Superalloys 1997, E.A. Loria, ed., TMS, Warrendale, PA, 1997, pp. 141–52.
D.G. Evans and M. Fahrmann: in Superalloys 2004, K.A. Green, T.M. Pollock, H. Harada, T.E. Howson, R.C. Reed, J.J. Schirra, and S. Walson, eds., TMS, Warrendale, PA, 2004, pp. 507–15.
T.M. Pollock, W.H. Murphy, E.H. Goldman, D.L. Uram, and J.S. Tu: in Superalloys 1992, S.D. Antolovich, R.W. Sturdrud, R.A. MacKay, D.L. Anton, T. Khan, R.D. Kissinger, and D.L. Klarstrom, eds., TMS, Warrendale, PA, 1992, pp. 125–34.
J.A. Van Den Avyle, J.A. Brooks, and A.C. Powell: JOM, 1998, vol. 50 (3), pp. 22–55, 49.
W. Yang, W. Chen, K. Chang, S. Mannan, J. deBarbadillo, and K. Morita: in Superalloys 718, 625, 706 and Various Derivatives, E.A. Loria, ed., TMS, Warrendale, PA, 2001, pp. 113–40.
M.G. Worster: J. Fluid Mech., 1992, vol. 237, pp. 649–69.
W. Yang, W. Chen, K-M. Chang, S. Mannan, and J. deBarbadillo: Metall. Mater. Trans. A, 2001, vol. 32A, pp. 397–406.
Z. Long, X. Liu, W. Yang, K.-M. Chang, and E. Barbero: Mater. Sci. Eng., A, 2004, vol. 386, pp. 254–61.
R. Mehrabian, M. Keane, and M.C. Flemings: Metall. Trans., 1970, vol.1, pp. 1209–20.
P. Auburtin, S.L. Cockcroft, A. Mitchell, and A.J. Schmalz: in Superalloys 1997, E.A. Loria, ed., 1997, TMS, Warrendale, PA, pp. 47–54.
R.A. Hobbs, S. Tin, and C.M.F. Rae: Metall. Mater. Trans. A, 2005, vol. 36A, pp. 2761–73.
S. Tin: Mater. Sci. Technol., 2009, vol. 25 (2), pp. 136–46.
G.A. Knorovsky, M.J. Cieslak, T.J. Headley, A.D. Romig, and W.F. Hammetter.: Metall. Mater. Trans. A, 1989, vol. 20A, pp. 2149–58.
Y. Du, S. Liu, Y.A. Chang, and Y. Yang: CALPHAD, 2005, vol. 29, pp. 140–48.
P.K. Sung and D.R. Poirier: Metall. Mater. Trans. A, 1999, vol. 30A, pp. 2173–81.
W.J. Boettinger and U.R. Kattner: Metall. Mater. Trans. A, 2002, vol. 33A, pp. 1779–94.
R.I. Wu and J.H. Perepezko: Metall. Mater. Trans. A, 2000, vol. 31A, pp. 497–501.
M. Rettenmayr and M. Buchmann: Mater. Sci. Forum, 2006, vol. 508, pp. 205–10.
H.B. Dong, M.R.M. Shin, E.C. Kurum, J.D. Hunt, and H. Cama: Metall. Mater. Trans. A, 2003, vol. 34A, pp. 441–47.
J. Miettinen: Metall. Mater. Trans. A, 1992, vol. 23A, pp. 1155–70.
P.D. Garn: J. Therm. Anal., 1975, vol. 7, pp. 593–99.
N. D’Souza, M. Lekstrom, and H.B. Dong: Mater. Sci. Eng., A, 2008, vol. 490, pp. 258–65.
J.N. DuPont, C.V. Robino, J.R. Michael, M.R. Notis, and A.R. Marder: Metall. Mater. Trans. A, 1998, vol. 29A, pp. 2785–96.
M.N. Gungor: Metall. Mater. Trans. A, 1989, vol. 20, pp. 2529–33.
M.C. Flemings, D.R. Poirier, R.V. Barone, and H.D. Brody: J. Iron Steel Inst., 1970, vol. 208, pp. 371–81.
J.E. Hillard: Report No. 61-RL-2652M, General Electric Co. Research Laboratory, Schenectady, NY, 1961.
J. Sarreal and G. Abbaschian: Metall. Trans. A, 1986,vol. 17A, pp. 2063–73.
M.C. Flemings: Solidification Processing, McGraw-Hill, New York, NY, 1974, pp. 34–36
S. Tin, T.M. Pollock, and W. Murphy: Metall. Trans. A, 2001, vol. 32A, pp. 1743–53.
W.D. Cao, R.L. Kennedy, and M.P. Willis: in Superalloys 1991, E.A. Loria, ed., TMS, Warrendale, PA, 1991, pp. 147–60.
W Yang, W. Chen, and K.-M. Chang: Annual Report for Research Project, West Virginia University, Morgantown, WV, 1999, pp. 15–16.
Acknowledgments
One of the authors (JV) acknowledges the financial support received by the Fulbright/Colciencias y el Departamento Nacional de Planeación (DNP) scholarship as well as the support from the Universidad del Valle.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 2, 2009.
Appendix
Appendix
The MatLab function code used to fit the experimental EDS data to the Scheil equation for microsegregation is included here. It calculates the error function defined in Eq. [5] and returns the partition coefficient that minimizes the error, i.e., the best-fit partition coefficient. The input information required are the ordered vector of original EDS measurements C s (according to segregation tendency), the original content of the alloying element in the parent melt C 0, the assumed minimum apparent solid fraction f smin (to make up for the low probability of hitting the real dendrite core), and the maximum reached apparent solid fraction before quenching f smax. An arbitrary data set is included for exemplification purposes.
Code to Determine Partition Coefficients from Fitting to Scheil Equation, Created by Jairo A. Valdes Ortiz, Department of Mechanical and Aerospace Engineering, West Virginia University, Morgantown, WV, Fall 2007
-
%
-
% Function Syntax
-
% partcoeff(Cs,Co,fmin,fmax)
-
% Cs is a vector containing the EDS data
-
% Co is the Melt Composition (Parent alloy)
-
% fsmin is the Apparent Solid Fraction at the Estimated Core Dendrite
-
% fsmax is Maximum Reached Apparent Solid Fraction Determined by Metallographic Analysis
-
function partcoeff(Cs,Co,fsmin,fsmax)
-
% Example Data
-
%% Original Data Collected by EDS
-
% Cs = [0.95, 1, 1, 1.03, 1.05, 1.06, 1.07, 1.19, 1.2, 1.25, 1.25, 1.25, 1.26, 1.31, 1.37, 1.44, 1.5, 1.64];
-
%% Initial value of Parent Composition [wt%]
-
% Co = 2.06;
-
%% Apparent Solid Fraction at the Estimated Core Dendrite
-
% fsmin = 0.05;
-
%% Maximum Reached Apparent Solid Fraction Determined by Metallographic Analysis
-
% fsmax = 0.9;
-
% Number of Data Points Entered
-
ndata = length(Cs);
-
% Estimated Values of the Apparent Solid Fraction
-
fs = linspace(fsmin,fsmax,ndata);
-
% Plotting original data of Apparent Solid Fraction vs. Solid Composition (Measured)
-
figure(1); grid on; hold on; title(‘Original data for Apparent Solid Fraction vs. Solid Composition (Measured)’);
-
xlabel(‘Apparent Solid Fraction’); ylabel(‘Solid Composition (Measured) [wt%]’);
-
plot(fs,Cs,‘ro’);
-
% Possible values for the Partition Coefficient
-
k = (0:0.001:1.5);
-
n = length(k);
-
% Error function to determine the Partition Coefficient
-
% yk = (Cs − k*Co*(1 − fs)^(k − 1))^2
-
yk(1:n) = 0;
-
for j=1:n
-
yk(j) = 0;
-
for i=1:length(fs)
-
yk(j) = yk(j) + (Cs(i) − k(j)*Co*(1 − fs(i))^(k(j) − 1))^2;
-
-
end
-
-
end
-
% Results
-
[ykmin,id] = min(yk);
-
kmin = k(id);
-
disp(‘Best fit Partition Coefficient’);
-
disp(kmin)
-
figure(2); grid on; hold on;
-
xlabel(‘partition coefficient’); ylabel(‘fitting error [wt%^2]’)
-
plot(k,yk,‘b’); plot(kmin,ykmin,‘rx’);
Rights and permissions
About this article
Cite this article
Valdes, J., Shang, SL., Liu, ZK. et al. Quenching Differential Thermal Analysis and Thermodynamic Calculation to Determine Partition Coefficients of Solute Elements in Simplified Ni-Base Superalloys. Metall Mater Trans A 41, 487–498 (2010). https://doi.org/10.1007/s11661-009-0132-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-009-0132-7