Abstract
This study aimed to estimate arsenic (As) and iron (Fe) content in tubewell water (n = 58) in primary educational institutions and subsequently assess the health risks to school-going children. Results described that the As concentration ranged between 0.002 and 0.994 mg L−1 with an average value of 0.044 mg L−1; which exceeded the World Health Organization (WHO) provisional guideline value of 0.01 mg L−1. Similarly, the Fe content varied from 0.05 to 10 mg L−1 averaging to 2.84 mg L−1. Samples of 55.17% contained a greater As concentration than 0.01 mg L−1 and 18.97% greater than Bangladesh drinking water quality (BDWQ) standard of 0.05 mg L−1, respectively. Meanwhile, 75.86% of samples contained a higher Fe concentration than the maximum Bangladesh permissible limit of 1 mg L−1. Health risk assessment indicated that girls are more vulnerable than boys are. The average hazard quotients (HQs) for As intake through drinking water were 6.01 ± 17.85 and 7.41 ± 22.03 for boys and girls, respectively, implying non-carcinogenic health risks to both genders. The HQs for Fe intake were less than threshold value of 1 indicating no health issues may arise from Fe intake alone. However, consumption of As and Fe may trigger health risks to students as indicated by the hazard index (HI), which was higher than 1. The average cancer risk (CR) values for both boys (0.0027 ± 0.008) and girls (0.0033 ± 0.0099) exceeded the threshold limit of 10–6–10–4, suggesting a possibility of lifetime cancer risks to the school-going children. Consequently, school authorities should find alternative ways to ensure safe drinking water for school-going children to avoid possible cancer and non-cancer health risks through consumption of As-poisoning water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater, which is collected through tubewells (TW) and for this reason known as tubewell water is the primary source of safe drinking water for 97% of rural inhabitants in Bangladesh (UNICEF 2021). However, the increased use of groundwater resources, natural processes like leaching and weathering, and anthropogenic inputs including mining and industrial activities have seriously endangered groundwater quality (Galhardi and Bonotto 2017; Islam et al. 2017a; Smedley and Kinniburgh 2002). Several contaminants affect water quality but in Bangladesh, trace elements, particularly arsenic (As) and iron (Fe) affect the groundwater quality the most (Fendorf et al. 2010; Nickson et al. 2000). This is due to the natural release of As into the aquifer through reductive dissolution of arsenic-rich iron oxyhydroxide (Nickson et al. 2000; Saha et al. 2020)
Arsenic is a carcinogenic element and exposure to it from drinking water causes various diseases including cancer (IARC 2018). Chronic exposure to As causes hepatocellular carcinoma, melanosis, hyperkeratosis, diabetes mellitus, hypertension, cancers of bladder, skin and lungs, cirrhosis, liver fibrosis,, IQ impacts in children, cardiovascular systems, endocrine disruption and parenchymal cell damage (Gupta et al. 2020; He et al. 2014; Rahman et al. 2009; Xu et al. 2021). Drinking water containing as and Fe can endanger the health of adults and children. Parkinson′s and Alzheimer′s diseases are caused by as exposure (De Vizcaya-Ruiz et al. 2009). Yadav et al. (2011) revealed that as exposure may cause impaired hearing, learning, and sleeping. Furthermore, a different dimensional impact was found for As that sulfur-containing protein can react with as(III) to form products that will cause biological malfunction (Smith et al. 2000; Wang and Wai 2004).
In contrast to As, Fe is one of the elements essential for human health, specifically for hemoglobin, myoglobin, and a number of enzymes. However, a high concentration of Fe causes vomiting, and diarrhea, with a subsequent effect on the cardiovascular and central nervous systems, liver, kidney, and blood in humans (Goldhaber 2003). Consequently, several studies were conducted on groundwater quality monitoring and estimation of several trace elements in groundwater in Rangpur division. Hydro-chemical evaluation by measuring major cations and physicochemical analysis of groundwater from Rangpur and Dinajpur districts indicated that groundwater can be utilized for drinking and domestic purposes (Saha et al. 2019). However, trace elements in groundwater from Rangpur district indicated that the average Fe concentration was higher and As concentration was smaller than the recommended limits published by the WHO and Bangladesh authorities (Islam et al. 2019). The study detected that As exposure from drinking water may pose lifetime cancer risks to the population in the study area, where children are at higher risk (Islam et al. 2019). Additionally, one study conducted in the Dinajpur district found that Fe concentration in groundwater surpassed the WHO and Bangladesh standard limits (Habib et al. 2020). In their study, they did not estimate As in groundwater.
Although several studies were conducted on groundwater pollution from trace elements in northern Bangladesh, none focused on groundwater contamination from trace elements, particularly As and Fe in the tubewells on primary school premises. In contrast to northern Bangladesh, several analyses done in southern and south-western Bangladesh have concentrated on trace element estimation and health risk assessment of trace elements through drinking water from the tubewells in primary schools. For example, in the southwestern Bangladesh district of Magura, As and Fe concentrations in tubewell water from primary schools were estimated (Rahman et al. 2016). It emerged that the concentrations of As and Fe were higher than the WHO provisional guideline value and Bangladesh drinking water quality (BDWQ) standard (Rahman et al. 2016). Tubewell water from primary schools in Satkhira district in southern Bangladesh revealed that about 49% and 45% tubewell water samples contained higher Fe and As content, respectively, than what the guidelines recommended (Rahman and Hashem 2019).
In many As polluted areas around the world, for example, in Sindh province of Pakistan, As concentration in drinking water was higher than 10 μg L−1 in most tubewells, and one health risk assessment model indicated that the children were at risk of chronic As toxicity in the future (Baig et al. 2016). Similar findings were reported for school-going children in Punjab, Pakistan (Murtaza et al. 2020). Furthermore, Rahman et al. (2021c) and Gul et al. (2020) reported that groundwater in Jessore and Multan districts is not fit for human consumption due to excessive As contamination and high school students are at risk of experiencing chronic and carcinogenic health risks. Moreover, in West Bengal, India, a study revealed that school children in As exposed areas are at a high risk of As exposure through the consumption of contaminated drinking water (Joardar et al. 2020). Furthermore, the average As concentration in the drinking water from the exposed area was 7 times higher than 10 μg L−1 (Joardar et al. 2021).
It should be noted that the tubewells in primary schools are the only source of drinking water for the students during school hours since children do not usually carry drinking water from home. A substantial time is spent in schools and the students drink about 1 L of water during school time, which may cause ingestion of a substantial amount of As and Fe. Excessive intake of As and Fe may cause several diseases as discussed earlier. Children are more vulnerable than adults as they have less developed respiratory, immune, reproductive, central nervous, and digestive systems (Burtscher and Schüepp 2012; Madureira et al. 2016; Salvi 2007). Consequently, a health risk assessment model has become an important tool in determining human health risks for trace elements intake. This technique has been widely used to determine probabilistic health risks to the population. Even in the current study area, some of the studies used this technique to determine probabilistic health risks to the general population and indicated that children have a great health risk susceptibility to the polluted groundwater in Setabganj sugar mill area (Hossain et al. 2020), females in Rangpur city area were more susceptible to the health risk from Fe intake than the males (Hauque et al. 2021), and children were at higher cancer and non-cancer risks than adults in Rangpur district from drinking groundwater (Islam et al. 2019). However, none of the studies in Rangpur division focused on As and Fe content in drinking water in primary schools and estimated the health risks to the students from drinking water during the school period.
With these issues in mind, for the first time, the present study examined the quality of drinking water of primary schools in different upazilas (sub-districts) of eight districts in northern Bangladesh (Rangpur division). Monitoring was done to quantify As and Fe concentrations using atomic absorption spectrophotometer (AAS) and compared with corresponding national and international provisional safe limits in drinking water. In addition, human health risks were estimated using the United States environmental protection agency (US-EPA) model for school-going children in the study area.
2 Materials and methods
2.1 Study area
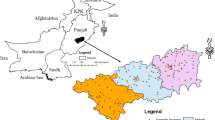
The study was conducted in the north-western Bangladesh area known as Rangpur division. The study area consists of eight districts, namely Rangpur (R), Dinajpur (D), Thakurgaon (T), Panchagarh (P), Nilphamari (N), Lalmonirhat (L), Kurigram (K), and Gaibandha (G) (Fig. 1). The study area is in fact an alluvial plain with an elevation of 32 m above sea level. The elevation rises from the Pleistocene terraces sloping towards the south and southeast (DPHE/BGS 2001; Rahman and Islam 2019). In Rangpur division, most of its areas are composed of alluvial recent soil and the rest are Barind clay. There are two types of aquifers, namely the upper shallow aquifer in the uplift zone of Rangpur Saddle and the lower aquifer. Coarse-grained sands with widespread gravels are present in the Late Pleistocene to Early Holocene aquifer, while Dupi Tila sand formation is overlayed by the thick silty clay formation of the Pleistocene period. It has formed the Plio-Pleistocene aquifer in the study area (Islam et al. 2019).
2.2 Sampling
The drinking water samples were collected from the 58 pre-selected tubewells of primary schools from each sub-district of eight districts (Fig. 1). The water samples were collected directly from the tubewells after purging for at least 20 min in the prewashed high-density polyethylene (HDPE) bottles. Then samples were labeled as D1–D13 for Dinajpur, G1–G7 for Gaibandha, K1–K9 for Kurigram, L1–L5 for Lalmonirhat, N1–N6 for Nilphamari, P1–P5 for Panchagar, R1–R8 for Rangpur, and T1–T5 for Thakurgaon district. Before analysis, the samples were filtrated through a millipore cellulose membrane (0.45 μm) in bottles and acidified with 1% nitric acid (HNO3). All samples were kept in a refrigerator at 4 ºC until required for laboratory analysis (Mohana et al. 2020).
2.3 Reagents and laboratory analysis
All stock solutions were prepared from the analytical reagents (AR). Freshly prepared double de-ionized distilled water was used in all experiments. Arsenic standard solutions were obtained from Fluka-Analytical Switzerland. 20% potassium iodide (Sigma-Aldrich, USA) solution was used to reduce As(V) to AS(III). Arsenic tri hydride (AsH3) generation was done with 5 M HCl (Sigma-Aldrich, USA), and 0.6% sodium boro hydride solution (Sigma-Adrich, USA).
The As and Fe contents were analyzed by an atomic absorption spectrophotometer following the American Public Health Association (APHA) method (APHA 2012). Firstly, the calibration curve was established using the working standard solutions from different concentrations of the certified reference solutions. The As content was measured by the hydride vapor generation (HVG) method, where argon served as the carrier gas and recordings were taken at the wavelength of 193.7 nm (Rahman et al. 2016). The Fe content was measured using direct IR acetylene flame at a wavelength of 248.3 nm (Rahman et al. 2019).
2.4 Human health risk assessment
The health risk assessment estimates a population's health to what extent would be at risk of drinking contaminated water. The health risks from non-carcinogenic and carcinogenic exposure arising from the intake of arsenic (As) and iron (Fe) were considered. The US-EPA recommended method was used for health risk assessment, which provides a qualitative estimate of health risks based on provisional standard values. The human non-carcinogenic health risk was evaluated on the basis of chronic daily intake (CDI) of metals and hazard quotient (HQ) for both boys and girls. If the HQ is higher than the threshold value equal to 1, it is assumed that the population might experience non-cancer health symptoms from drinking contaminated water. However, there is no guideline value for the CDI, which only indicates the daily consumption content of pollutants by the people. In addition, carcinogenic risk (CR) was calculated for As, since only As has an oral slope factor value. The threshold limit of CR is 10–6–10–4 and if the value is greater than this range, it is expected that the consumers might develop cancer in their lifespan. TW water is used for cooking, drinking, and bathing, but in school areas, it is primarily used for drinking purposes. Therefore, in human health risks estimation, only water ingestion was considered as the primary exposure route to the children:
where C is the concentration of As and Fe in water (mg L−1); IR is the ingestion rate of water (1 L day−1) for school-going children during school time (Rahman et al. 2021a); EF represents the exposure frequency (230 days/year); ED is exposure duration (5 years); AT is the average exposure time (1150 days); and BW denotes the body weight for 6 to 10 years school children. It is noted that the mean body weights of boys and girls are approximately 24.68 and 20 kg, respectively (NCHS 2001). In this study, we have considered 1.0 L water consumption during school hours (about 3–4 h/day) to estimate health risk, although children usually consume 2.1 L of water daily (Hossain et al. 2013). This is because, there are two shifts of school time in primary schools in Bangladesh: the morning shift (9:15 am–12:00 pm for class I to II students) and day shift (12:15 pm– 4:15 pm for class III to V students), which are subsequently after breakfast and lunch when children drink more water than other time in the day, respectively. Exposure duration was considered based on school opening days annually. Also, water ingestion was considered the major exposure route since students only drink water during the average schooling day; they do not take a bath which is the dermal and inhalation exposure route.
The HQ was estimated using the following equation (US-EPA 2002, 2005):
where RfDo is the oral reference dose of As (0.0003 mg kg−1 day−1) and Fe (0.7 mg kg−1 day−1) (US-EPA 2005). When the value of HQ < 1, the exposed population is safe from certain harmful effects of trace metals. If HQ > 1, then this may pose adverse health effects on the exposed population.
Hazard index (HI) is the sum of HQ of As and Fe. It is stated as:
The CR is considered by means of the subsequent formula (US-EPA 2019):
where SF is the oral slope factor for As (mg/kg/day)−1 and for As, the value of SF is 1.5 (mg kg−1 day−1)−1 (US-EPA 2019).
3 Results and discussion
3.1 As and Fe in drinking water
Figures 2 and 3 depict the concentrations of As and Fe in groundwater in Rangpur division (north-western part of Bangladesh). The concentration of As in the water samples ranged from 0.002 to 0.994 mg L−1 with an average value of 0.044 mg L−1. The highest As content (0.994 mg L−1) was discovered in Pirgacha sub-district in Rangpur (R-2), while the smallest was reported in Parbatipur sub-district in Dinajpur (D − 10) (Fig. 1). The WHO provisional guideline value for As in drinking water is 0.01 mg L−1 (WHO 2017) while for Bangladesh, it is 0.05 mg L−1 (BSB/UNICEF 2011; ECR 1997). The results indicated that about 55.17% (32 out of 58) samples contained a higher As concentration than the WHO guideline value, whereas, about 18.97% (11 out of 58) groundwater contained more than the Bangladesh drinking water quality standard limit of 0.05 mg L−1. The average concentration of As (0.044 mg L−1) in the study area was about 4.5 times higher than the WHO standard but slightly lower than the BDWQ limit (Supplementary Table S1).
Among the 8 districts, the highest As concentration was detected in Rangpur district (average: 0.146 mg L−1; range: 0.005–0.994 mg L−1), while the lowest was found in Lalmonirhat district (Average: 0.007 mg L−1; range: 0.005–0.01 mg L−1) (Table 1). Average As concentration in groundwater in Lalmonirhat (0.007 mg L−1) and Thakurgaon (0.009 mg L−1) districts were below the WHO guideline value of 0.01 mg L−1, whereas, in other districts average As concentrations were higher than the WHO standard limit. Only groundwater from Rangpur and Kurigram districts showed a higher As concentration than the BDWQ standard of 0.05 mg L−1 (Table 1). Previous studies carried out in Rangpur district found an average of 0.009 mg L−1 As in groundwater (Islam et al. 2019), which is about 16 times less than what this study reported in Rangpur district (0.146 mg L−1). In addition, the average As concentrations in groundwater found in Dinajpur (0.014 mg L−1), Gaibandha (0.025 mg L−1), Kurigram (0.093 mg L−1), Nilphamari (0.014 mg L−1), and Panchagar (0.011 mg L−1), were higher than previous research in Rangpur district by Islam et al. 2019 (0.009 mg L−1) (Table 1). However, the average As concentration in Lalmonirhat (0.007 mg L−1) and Thakurgaon (0.009 mg L−1) districts was similar to past studies (0.009 mg L−1) in Rangpur district (Islam et al. 2019). Besides, average As concentrations in tubewell water from Dinajpur (0.014 mg L−1) and Nilphamari districts (0.014 mg L−1) were similar to Kushtia district (0.017 mg L−1) (Rahman and Rahaman 2018), and almost 10 times lower than Narail district (0.091 mg L−1) as reported by Mohana et al. (2020). This inter-district difference in As concentration was probably due to the difference in geological formation and local anthropogenic activities in Rangpur division.
Taking into account tubewell water from primary schools, a large difference (about 2–3 times higher) was observed in As concentration between southwestern and northwestern Bangladesh (this study area). In this study, the average As concentration was 0.044 mg L−1, while in Magura district it was 0.016 mg L−1 (Rahman et al. 2016), and in Satkhira district it was 0.026 mg L−1 (Rahman and Hashem 2019). Although the reported As concentration is lower than the present study, it is still higher than the WHO provisional guideline limit of 0.01 mg L−1 (WHO 2011).
Generally, there is a difference in geological characteristics over a large area. So, the difference in As concentration in inter-region contexts might be linked to the hydrogeology of the study area, which is largely related to the depth of a tubewell. In the present study area, there are two types of aquifers, namely the upper shallow aquifer in the uplift zone of Rangpur Saddle and the lower aquifer. The shallow aquifer is characterized by the presence of sands with widespread gravels that formed during the Late Pleistocene to Early Holocene. In contrast, the deep aquifer is characterized by Dupi Tila sand formation overlaid by the thick silty clay that was formed during the Pleistocene (Islam et al. 2019). Meanwhile, the geology of the south-western region is characterized by Pleistocene-Modhupur clay underlying the fluvial-deltaic sediment of the Holocene. The Holocene sediment is mainly composed of quartz with some plagioclase and potassium feldspars, with some fragments of volcanic, metamorphic, and sedimentary rocks (Halim et al. 2010; Uddin and Lundberg 1999). The aquifer in southwestern Bangladesh has three stages, namely, a semiconfined, shallow Holocene aquifer (extend 100 m below ground level) that is vertically separated from two Pleistocene aquifers (extend 200 and 300 m below ground level) (Burgess et al. 2011; Rahman et al. 2011). In the Holocene aquifer, there is a higher level of groundwater As, which was caused by the natural release of As into the aquifer through reductive dissolution of arsenic-rich iron oxyhydroxide (Nickson et al. 1998; Saha et al. 2020). Nevertheless, anthropogenic activities are also responsible for the release of As in groundwater.
It is important to note that in north-western Bangladesh, tubewell depth is shallower (about 10–53 m) than in the south-western part (about 13–220 m) (Islam et al. 2019; Rahman and Hashem 2019). It was reported that As concentrations in shallow, intermediate and deep tubewells were 0.041, 0.0253, and 0.02138 mg L−1, respectively, which is a gradual decline in As concentration from shallow to deep tubewell (Ahmed et al. 2019; Mohana et al. 2020). Consequently, it is apparent that tubewells in southwestern Bangladesh collect water from the Pleistocene aquifer that is in an oxidized state, which reduces the mobility of As in groundwater. However, in northwestern Bangladesh, tubewells do penetrate into the Holocene aquifer having the reducing condition that facilitates mobilization of As into groundwater. A recent study on hydrogeochemistry also indicated that As concentration reduces with increase in aquifers depth as the condition of the aquifer changes from oxidizing to a reducing state. The reducing state was confirmed by the dominance of some ions, for example, 84% of samples of the Holocene aquifer showed the dominance of As (III) species with Fe2+ and Mn2+ (Saha et al. 2020). Furthermore, a higher As concentration was observed in this area.
The BDWQ standard of Fe in drinking water is 0.3–1.0 mg L−1 (BSB/UNICEF 2011; ECR 1997), while there is no WHO guideline value for Fe in drinking water (WHO 2017). In this study, Fe content ranged between 0.05 and 10.0 mg L−1 with an average Fe content of 2.84 mg L−1 (Supplementary Table S1). The average value for Fe in this study was 2.84 mg L−1, which is about 3 times higher than the maximum permissible limit of 1 mg L−1 (ECR 1997). It was observed that about 75.86% (44 out of 58) of drinking water sources contained Fe content higher than the BDWQ maximum permissible value of 1 mg L−1 (Supplementary Table S1). The highest Fe concentration was found in Boda, Panchagar (10 mg L−1), while the lowest was reported in Birgang, Dinajpur (0.05 mg L−1) (Fig. 3). There was no large difference in average Fe concentration in different districts in Rangpur division. The average Fe concentrations were 1.7, 2.22, 3.12, 1.62, 3.45, 5.08, 3.98, and 2.61 mg L−1 in Dinajpur, Gaibandha, Kurigram, Lalmonirhat, Nilphamari, Panchagarh, Rangpur, and Thakurgaon, respectively (Table 1). However, mean Fe concentrations were higher than the maximum permissible limit of the Bangladesh standard. A previous study in Rangpur district found 7.73 mg L−1 Fe in groundwater (Islam et al. 2019), which is more than double the present study’s findings in Rangpur district (3.45 mg L−1). However, in Dinajpur district Fe concentration was 1.58 mg L−1 (Habib et al. 2020), which is almost close to what was found in Dinajpur district, i.e. 1.7 mg L−1. In Kushtia district (western part of Bangladesh), the average Fe concentration was 0.25 mg L−1 (Rahman and Rahaman 2018), which is lower than what this study reported for eight districts (Table 1). The Fe concentration in the south-western part of Bangladesh was reported to 1.25, 2.17, 2.18, and 3.6 in Magura, Narail, Khulna, Satkhira district, respectively in past studies (Mohana et al. 2020; Rahman et al. 2016, 2021a, 2019). In line with As, the higher level of Fe in groundwater may be attributed to its natural origin in soil, rock, and minerals that undergo dissolution through interaction with groundwater. The reducing condition of the Holocene aquifer, a higher concentration of Fe and As coupled with the basic nature (pH = 6.9–8.8) of groundwater in the study area (Islam et al. 2017b) suggest the natural release of Fe from Fe and Mn oxyhydroxides (Ravenscroft et al. 2015). Additionally, the prevailing condition of the aquifer might have assisted the release of As-bearing Fe and Mn-oxyhydroxides from the organic-rich sediment as follows (Nickson et al. 2000; McArthur et al. 2001; Anawar et al. 2004; Islam et al. 2004):
4FeOOH + CH2O (organic matter) + H2CO3 = 4Fe2+ + 8HCO32− + 6 H2O.
2MnO2 + CH2O (organic matter) + H2CO3 = 2Mn2+ + 4HCO32− + 2 H2O.
3.2 Human health risk assessment
In this study, health risks to school-going children were estimated for school time only in terms of non-carcinogenic and carcinogenic health risks. The non-carcinogenic health risk was assessed based on chronic daily intake (CDI) of metals, hazard quotient (HQ), and hazard index (HI) for both boys and girls. In addition, carcinogenic risk (CR) was calculated for As, since only As has oral slope factor value between As and Fe.
For each sampling point, the CDI values of As and Fe from the consumption of drinking water in the case of both boys and girls are presented in Fig. 4. Descriptive statistics for CDI of As and Fe in eight districts as well as in Rangpur division are provided in Table 2. In Rangpur division, CDIs of As were 0.0018 ± 0.0054 and 0.0022 ± 0.0066 mg kg−1 day−1 for boys and girls, respectively. The lowest CDI of As was observed for boys (0.0003 ± 0.0001 mg kg−1 day−1) in Lalmonirhat district, while the highest was observed for girls (0.0073 ± 0.0172 mg kg−1 day−1) in Rangpur district (Table 2).
CDI of Fe was higher than As and the CDIs of Fe were 0.1151 ± 0.1014 and 0.1420 ± 0.1252 mg kg−1 day−1 for boys and girls, respectively in Rangpur division (Table 2). The highest CDI of Fe was observed for girls (0.2540 ± 0.1590 mg kg−1 day−1) in Panchgar district, while the lowest was observed for boys (0.0655 ± 0.0262) in Lalmonirhat district. This outcome indicated that the CDI of Fe is higher than As due to the higher concentration of Fe than As in groundwater. As well, the girls ingest more As and Fe than the boys, this being caused by the different body weights of boys and girls. The boys are heavier than the girls are and this guides their CDI of As and Fe through drinking water.
The HQ and HI for each primary school in the study area are depicted in Figs. 5 and 6.
It is apparent that HQ was higher for As intake than Fe. The average HQs for As intake through drinking water were 6.01 ± 17.85 and 7.41 ± 22.03 for boys and girls, respectively (Table 3), which is 6 and 7 times higher than threshold value of 1, indicating severe non-cancer health risks to the students. In every district the average HQ from As intake for boys and girls was higher than 1, except for boys (0.99 ± 0.31) in Lalmonirhat district; however, it was very close to 1 (Table 3). The highest HQ from As intake was noted in Rangpur district for both boys (19.74 ± 46.37) and girls (24.35 ± 57.22). Added to this, the highest HQ was observed for sampling point R-2 (boys: 134.25 and girls: 165.67), which is in Rangpur district indicating the highest non-cancer risks to the students of this school (Fig. 5). Referring to Fe intake, the average HQs for boys and girls in Rangpur division were 0.15 ± 0.14 and 0.2 ± 0.18, respectively, and in all of the eight districts HQs for Fe intake were lower than threshold value of 1. Suggested here is that there is no non-cancer risks to the students.
The HI is the sum of HQ for As and Fe intake from drinking water. Since HQ from As is dominant over Fe, the HI for boys and girls follows the similar trend of HQ for As. In this study, the average values of HI for boys and girls were 6.16 ± 17.87 and 7.61 ± 22.1, respectively, which is similar to HQ for As intake (Table 3). In south-western Bangladesh, HQ for As intake with reference to school going children was also higher than 1, but it was much lower than what Rahman and Hashem (2019) documented. They reported the highest HQ for As in Kaligonj, Satkhira (HQ = 6.34), which is about 3–4 times lower than the highest HQ value found in this study. Therefore, the exposed population is at a level of serious health concern, for example, it can cause mild respiratory effects, such as dry cough and asthma in females, but pronounced effects in males exposed to high concentrations of As in drinking water in the early stages of life (Khan et al. 2020). Children are likely to experience respiratory symptoms, pediatric pneumonia, cardiovascular diseases, and cancers of the bladder, lung, and kidneys (Osorio-Yáñez et al. 2013; Osorio-Yáñez et al. 2015; Rahman et al. 2013; Smith et al. 2013), neurological disorders and altered neurobehavioral development in adolescents in the study area (Osorio-Yáñez et al. 2013; Yorifuji et al. 2016).
Figure 7 shows the estimated CR value of As for both school-going boys and girls. Besides, CR of As for each district in Rangpur division is provided in Table 4. In Rangpur division, it was observed that average CR values for both the boys (0.0027 ± 0.008) and girls (0.0033 ± 0.0099) exceeded the threshold limit of 10–6–10–4 provided by US-EPA (USEPA 2015). The CRs were 2.7 and 3.3 times higher than the highest threshold limit of 10–4. Besides, average CR values for each district in Rangpur division exceeded the threshold value. The highest CR was found for girls (0.011 ± 0.0258) in Rangpur district, while the lowest was found for boys (0.0004 ± 0.0001) in Lalmonirhat district (Table 4). Based on this, it is evident that As in drinking water may cause lifetime cancer risk, particularly, skin, liver, lung, kidney, and bladder cancer to the primary school students in the study area (Smith et al. 1992).
3.3 Correlation between As and Fe
There were 26 water samples out of 58 having As concentration < 0.01 mg L−1. Among 26 samples having As < 0.01 mg L−1, 25 samples had Fe concentration higher than maximum contamination level (MCL) of 0.3 mg L−1 and 22 samples had a concentration higher than BDWQ level of 1 mg L−1. No significant correlation between As vs. Fe (r2 = 0.0479) was observed in tubewell water when the As concentration < 0.01 mg L−1 in the studied area (Fig. 8A). Similarly, there was 32 water samples having As concentration > 0.01 mg L−1, where 30 water samples had Fe concentration higher than MCL by US-EPA (2017) and 23 water samples had concentration higher than BDWQ level of 1 mg L−1. No significant correlation was found between As vs. Fe (r2 = 0.0105) was observed in tubewell water when the As concentration > 0.01 mg L−1 (Fig. 8B).
4 Conclusions
The tube well water samples were collected from eight districts of Rangpur Division (north-western part of Bangladesh) to understand the pollution level and possible health risks of As and Fe to the school-going children at a primary educational institution. It was observed that Rangpur is an extensively As polluted district among eight districts in Rangpur division. About 55.17% (32 out of 58) samples contained higher As concentration than the WHO provisional guideline value, whereas, about 18.97% (11 out of 58) groundwater contained higher than the BDWQ standard limit. A large difference in groundwater As content in north-western and south-western Bangladesh was observed, which was due to the depth of tubewells. Clearly, in north-western Bangladesh, tubewell depth is smaller regarding the penetration in the Holocene aquifer, which is reductive in nature and releases more As into groundwater. On the other hand, in southwestern Bangladesh, tubewells are deeper than is inserted into the Pleistocene aquifer, which is characterized by oxidization that releases the least As or suppresses the release of As into groundwater. It also emerged that Fe concentration was higher and about 75.86% (44 out of 58) drinking water sources contained more Fe than the BDWQ standard level of 1 mg L−1. Similar to As, the geological factors control the release of Fe in north-western Bangladesh.
Health risk assessment showed that the CDI of Fe is higher than As due to the higher Fe concentration in groundwater. In addition, regarding gender differences, the HQ and CR for girls were higher than the boys, so the girls are more prone to cancer and non-cancer risks. The HQ from As intake is higher than the threshold value of 1, while the HQ from Fe intake was much lower than the safe value. This is an indication that As may pose lifetime non-cancer risks to school-going children in the study area. Apart from this, there is a higher possibility of enduring lifetime cancer risks from drinking this contaminated water.
Therefore, this study suggests that it is necessary to drinking tubewell water in the investigated schools and find another way to remove As from drinking water. The Pleistocene aquifer which is a deeper aquifer is not a safe alternative source as it was observed that deeper aquifers contain less As, but nonetheless may cause lifetime cancer risks to children. For this reason, cost-effective arsenic iron removal plants (AIRPs) (Rahman et al. 2021b) and SONO filters (one type of efficient As-Fe removal plant) can be installed in school premises to reduce As and Fe from raw drinking water to a safer level.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author.
References
Ahmed N, Bodrud-Doza M, Islam ARMT, Hossain S, Moniruzzaman M, Deb N, Bhuiyan MAQ (2019) Appraising spatial variations of As, Fe, Mn and NO3 contaminations associated health risks of drinking water from Surma basin. Bangladesh Chemosphere 218:726–740
APHA American Public Health Association (APHA). Standard Methods for examination of water and wastewater (22nd ed.). . In, 2012. Washington DC: American Public Health Association,
Anawar HM, Akai J, Sakugawa H (2004) Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere 54:753–762
Baig JA, Kazi TG, Mustafa MA, Solangi IB, Mughal MJ, Afridi HI (2016) Arsenic exposure in children through drinking water in different districts of Sindh. Pak Biol Trace Elem Res 173:35–46
BSB/UNICEF (2011) Bangladesh national drinking water quality survey of 2009 Bangladesh Bureau of Statistics, Dhaka, Bangladesh. https://washdata org/sites/default/files/documents/reports/2019-06/Bangladesh-2009-MICS-water-quality-report pdf. Accessed 12 Aug 2019
Burgess WG, Hasan MK, Rihani E, Ahmed KM, Hoque MA, Darling WG (2011) Groundwater quality trends in the Dupi Tila aquifer of Dhaka, Bangladesh: sources of contamination evaluated using modelling and environmental isotopes. Int J Urban Sustain Develop 3(1):56–76
Burtscher H, Schüepp K (2012) The occurrence of ultrafine particles in the specific environment of children. Paediatr Respir Rev 13:89–94
De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME (2009) Biomarkers of oxidative stress and damage in human populations exposed to arsenic mutation research/genetic toxicology and environmental. Mutagenesis 674:85–92
DPHE/BGS (2001) Arsenic contamination of groundwater in Bangladesh Final report BGS Tech Rep WC/00/19 Keyworth British Geological Survey
ECR (1997) Environment Conservation Rules. E.C.R.-Schedule-3. Department of Environment & Forest Ministry, Bangladesh:205
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328:1123–1127
Galhardi JA, Bonotto DM (2017) Radionuclides (222 Rn, 226 Ra, 234 U, and 238 U) release in natural waters affected by coal mining activities in southern Brazil Water. Air Soil Pollut 228:1–19
Goldhaber SB (2003) Trace element risk assessment: essentiality vs toxicity. Regul Toxicol Pharmacol 38(2):232–242
Gul M, Mashhadi AF, Iqbal Z, Qureshi TI (2020) Monitoring of arsenic in drinking water of high schools and assessment of carcinogenic health risk in Multan. Pak Human Ecol Risk Assess Int J 26:2129–2141
Gupta R, Kumar P, Fahmi N, Garg B, Dutta S, Sachar S, Matharu AS, Vimaleswaran KS (2020) Endocrine disruption and obesity: A current review on environmental obesogens. Curr Res Green Sustain Chem 3:100009
Habib MA et al (2020) Simultaneous appraisals of pathway and probable health risk associated with trace metals contamination in groundwater from Barapukuria coal basin. Bangladesh Chemosphere 242:125183
Halim M et al (2010) Evaluation of processes controlling the geochemical constituents in deep groundwater in Bangladesh: spatial variability on arsenic and boron enrichment. J Hazard Mater 180:50–62
Haque M, Zahan RN, Reza S (2021) Iron in groundwater: quality evaluation, health risk, and spatial distribution in Rangpur City Corporation. Arab J Geosci 14(7):1–3
He J, Wang M, Jiang Y, Chen Q, Xu S, Xu Q, Jiang BH, Liu LZ (2014) Chronic arsenic exposure and angiogenesis in human bronchial epithelial cells via the ROS/miR-199a-5p/HIF-1 α/COX-2 pathway. Environ Health Perspect 122(3):255–261
Hossain MA et al (2013) Water consumption patterns and factors contributing to water consumption in arsenic affected population of rural West Bengal. India Sci Total Environ 463:1217–1224
Hossain SMS et al (2020) Assessing the groundwater quality and health risk: a case study on Setabganj sugar mills limited. Dinajpur Bangladesh Water Sci 34:110–123
IARC (2018) International Agency for Research on Cancer. Agents Classified by the IARC Monographs, Volumes 1–123. Lyon, France. https://monographs.iarc.who.int/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017a) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24:26350–26374
Islam ARMT, Shen SH, Bodrud-Doza M (2017b) Assessment of arsenic health risk and source apportionment of groundwater pollutants using multivariate statistical techniques in Chapai-Nawabganj district. Bangladesh J Geol Soc India 90:239–248
Islam ARMT, Bodrud-Doza M, Rahman MS, Amin SB, Chu R, Al Mamun H (2019) Sources of trace elements identification in drinking water of Rangpur district, Bangladesh and their potential health risk following multivariate techniques and Monte-Carlo simulation Groundwater for. Sustain Dev 9:100275
Joardar M, Das A, Mridha D, De A, Chowdhury NR, Roychowdhury T (2021) Evaluation of acute and chronic arsenic exposure on school children from exposed and apparently control areas of West Bengal. India Expos Health 13:33–50
Joardar M, Das A, Mridha D, De A, Chowdhury NR, Roychowdhury T (2021) Evaluation of acute and chronic arsenic exposure on school children from exposed and apparently control areas of West Bengal. India Expos Health 1:33–50
Khan MA et al. (2020) Prospective cohort study of respiratory effects at ages 14 to 26 following early life exposure to arsenic in drinking water Environmental Epidemiology 4
Madureira J, Paciência I, Rufo J, Severo M, Ramos E, Barros H, de Oliveira FE (2016) Source apportionment of CO2, PM10 and VOCs levels and health risk assessment in naturally ventilated primary schools in Porto. Port Build Environ 96:198–205
McArthur JM, Ravenscroft P, Safiulla S, Thirlwall MF (2001) Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour Res 37:109–117
Mohana A, Rahman MA, Islam MR (2020) Deep and shallow tubewell water from an arsenic-contaminated area in rural Bangladesh: risk-based status. Int J Energy Water Res 4:163–179
Murtaza B et al (2020) Hydrogeochemical investigation of arsenic in drinking water of schools and age dependent risk assessment in Vehari District Punjab Pakistan Multivariate analysis. Environ Sci Pollut Res 27:30530–30541
NCHS (2001) National Center for Health Statistics (NCHS). Data Table of Weight-for-age Charts [WWW Document]. https://wwwcdcgov/growthcharts/html_charts/wtagehtm#males. Accessed 6 2120
Nickson R, McArthur J, Burgess W, Ahmed KM, Ravenscroft P, Rahmann M (1998) Arsen Poisoning Bangladesh Groundw Nature 395:338–338
Nickson R, McArthur J, Ravenscroft P, Burgess W, Ahmed K (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Osorio-Yáñez C et al (2013) Carotid intima-media thickness and plasma asymmetric dimethylarginine in Mexican children exposed to inorganic arsenic. Environ Health Perspect 121:1090–1096
Osorio-Yáñez C, Ayllon-Vergara JC, Arreola-Mendoza L, Aguilar-Madrid G, Hernández-Castellanos E, Sánchez-Peña LC, Del Razo LM (2015) Blood pressure, left ventricular geometry, and systolic function in children exposed to inorganic arsenic. Environ Health Perspect 123(6):629–635
Rahman MA, Hashem MA (2019) Arsenic, iron and chloride in drinking water at primary school, Satkhira, Bangladesh. Phys Chem Earth Parts a/b/c 109:49–58
Rahman MS, Islam ARMT (2019) Are precipitation concentration and intensity changing in Bangladesh overtimes? analysis of the possible causes of changes in precipitation systems. Sci Total Environ 690:370–387
Rahman MM, Ng JC, Naidu R (2009) Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health 31:189–200
Rahman MT, Rahman MS, Quraishi S, Ahmad J, Choudhury T, Mottaleb M (2011) Distribution of heavy metals in water and sediments in Passur River Sundarban Mangrove Forest, Bangladesh. J Int Environ Appl Sci 6:537
Rahman MA, Kumar S, Lamb D, Rahman MM (2021c) Health risk assessment of arsenic, manganese, and iron from drinking water for high school children. Water Air Soil Pollut 232:1–13
Rahman A, Rahaman H (2018) Contamination of arsenic, manganese and coliform bacteria in groundwater at Kushtia District, Bangladesh: human health vulnerabilities. J water health 16(5):782–95
Rahman A, Hashem A (2016) Potable water quality monitoring of primary schools in Magura district, Bangladesh: children’s health risk assessment. Environ monit assess 188(12):1
Rahman M, Kumar S, Mohana AA, Islam R, Hashem M, Chuanxiu L (2019) Coliform Bacteria and trace metals in drinking water, southwest Bangladesh: Multivariate and human health risk assessment. Int j environ res 2:395–408
Rahman M, Islam M, Kumar S, Al-Reza SM (2021) Drinking water quality, exposure and health risk assessment for the school-going children at school time in the southwest coastal of Bangladesh. J Water Sanit Hyg Develop 11(4):612–28
Rahman MA, Kumar S, Bari AF, Sharma A, Rahman MM (2021) Efficiency of arsenic and iron removal plants (AIRPs) for groundwater treatment in rural areas of Southwest Bangladesh. Water 13(3):354
Rahman M et al. (2013) Increased childhood mortality and arsenic in drinking water in Matlab, Bangladesh: a population-based cohort study PloS one 8:e55014
Ravenscroft P, Burgess WG, Ahmed KM, Burren M, Perrin J (2005) Arsenic in groundwater of the Bengal basin, Bangladesh: distribution, field relations, and hydrogeological setting. Hydrogeol J 13:727–751
Saha N, Bodrud-Doza M, Islam AT, Begum BA, Rahman MS (2020) Hydrogeochemical evolution of shallow and deeper aquifers in central Bangladesh: arsenic mobilization process and health risk implications from the potable use of groundwater. Environ Earth Sci 79:1–18
Saha S, Reza AS, Roy MK (2019) Hydrochemical evaluation of groundwater quality of the Tista floodplain Rangpur, Bangladesh. Appl Water Sci 9:1–12
Salvi S (2007) Health effects of ambient air pollution in children. Paediatr Respir Rev 8:275–280
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl geochem 17(5):517–68
Smith AH et al (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78:1093–1103
Smith AH et al (2013) Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int J Epidemiol 42:1077–1086
Uddin A, Lundberg N (1999) A paleo-brahmaputra? subsurface lithofacies analysis of miocene deltaic sediments in the Himalayan-Bengal System, Bangladesh. Sediment Geol 123:239–254
UNICEF (2021) Better access to safe drinking water. https://www.unicef.org/bangladesh/en/better-access-safe-drinking-water. World Health Organization,
US-EPA (2019) United States-Environmental Protection Agency. 2019. Regional Screening Levels (RSLs) - Equations. Washington D.C., USA. https://www.epa.gov/risk/regional-screening-levels-rsls-equations (accessed 26.08.19).
US-EPA (2002) United State, Environmental Protection Agency, Region 9, Preliminary Remediation Goals.
US-EPA (2005) Guidelines for carcinogen risk assessment. Risk Assessment Forum, United States Environmental Protection Agency, Washington, DC. EPA/630/P-03/001F.
USEPA (2015) Risk Based Screening Table. Composite Table: Summary Tab 0615.
Wang JS, Wai CM (2004) Arsenic in drinking water—a global environmental problem. J Chem Edu 81(2):207
WHO (2011) Guidelines for Drinking-Water Quality, 4th edn. World Health Organization, Geneva
WHO (2017) Guidelines for drinking-water quality: incorporating the1st addendum World Health Organization, Geneva:415
Xu L et al (2021) Assessment of hypertension association with arsenic exposure from food and drinking water in Bihar. India Ecotoxicol Environ Saf 223:112572
Yadav RS et al (2011) Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology 32:760–768
Yorifuji T, Kato T, Ohta H, Bellinger DC, Matsuoka K, Grandjean P (2016) Neurological and neuropsychological functions in adults with a history of developmental arsenic poisoning from contaminated milk powder. Neurotoxicol Teratol 53:75–80
Acknowledgements
The authors are thankful to the authorities of all primary schools who allowed and helped the collections of water samples from the respective tube wells. The authors also acknowledge the DPHE Zonal Laboratory, Rangpur for providing facilities for trace element testing.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to publication
All authors read and approved the final manuscript.
Ethical approval
Not applicable in this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rana, M.S., Alam, M.J., Musa, M.A. et al. Groundwater arsenic poisoning in a primary educational institution: health risks to school-going children. Acta Geochim 41, 1069–1082 (2022). https://doi.org/10.1007/s11631-022-00563-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-022-00563-w