Abstract
Yam is an important staple in sub-Saharan Africa, but the availability of quality seed yam is majorly constrained by the low propagation ratio. This is because the propagating explant is limited to the tuber and nodal parts as yam rarely flowers. There are several reports of the use of somatic embryogenesis (SE) in the rapid propagation of different crop species and as a regenerative pathway in plant genetic engineering. However, SE deployment in yam is still at the protocol development stage. This review thus exploits the status of SE application in improving the yam propagation rate. This article reviews the potential of the various yam propagation techniques in rapidly multiplying disease-free yam with their propagating explants. The advantages SE offers are rapidly propagating yam, the factors to consider in the protocol optimization of SE application in rapidly multiplying different yam varieties, and as a platform for full utilization of genetic engineering in yam. The findings so far show that SE potentially offers a faster rate of propagating yam varieties. However, due to the differences in varietal endogenous hormonal and gene products, response to SE in yam is constrained by varietal specificity. Hence, the applicability of SE in yam is still at the protocol development state. This review, thus, presents the need for more research efforts to elucidate the molecular and phytochemical controlling mechanisms of SE in yam to improve the yam multiplication rate and lay an efficient platform for the exploitation of other biotechnological advancements in improving yam species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yam is one of the most valuable tuber crops in Nigeria that produces about 70% of the world’s output. However, Nigeria is not among the first 10 exporters of yam in the world, meaning that quantity produced could not satisfy the demand in the country. Several factors (biotic and abiotic) have been implicated in the scarcity of yam in the country with the availability of quality seed yam being the most important as it is significantly affected by the low propagation ratio 1:4. This low propagation ratio has limited yam cultivation and largely determines the dissemination rate of developed and improved varieties, and the expansion of yam cultivation as the time taken to mass-produce clean and quality seed yam is long (Otoo et al. 2016).
This problem of low propagation ratios has led to the development of different ex vitro propagation methods, such as the advanced yam minisett technique, rooted vine cutting and hydroponics systems (aeroponics and drip system hydroponics), and plant tissue culture (organogenesis: propagation from organs having pre-existing meristems) to improve yam production and availability (Aighewi et al. 2015). The above technologies have successfully improved the yam multiplication ratio from 1:3 obtained in the traditional methods of propagation (for example, whole tuber and milking methods) annually to 1:300 (Maroya et al. 2014) (Table 1). Despite the improvement, the availability of clean seed yam is largely dependent on the in vitro techniques. However, in vitro propagation techniques, like organogenesis and somatic embryogenesis, have been widely used to propagate different crop species with the use of organogenesis more optimized.
Why yam somatic embryogenesis?
Somatic embryogenesis has shown potential to improve the propagation of different crop species relative to other techniques. In yam, the advantages go beyond rapid multiplication, but they will create a pathway to improving yam through genetic transformation as the improvement of yam via conventional breeding is constrained by lack of flowering synchronization. However, the application of somatic embryogenesis in propagating yam is recent and less researched relative to organogenesis. This has caused yam seed companies to largely rely on organogenesis until somatic embryogenesis is well optimized for the exploitation of its full potential. To fully research and utilize somatic embryogenesis in yam improvement, it is important to understand somatic embryogenesis concepts and the factors controlling the system in crops as it will benefit from its full-scale adoption in yam propagation.
Factors influencing somatic embryogenesis
Factors, such as age of explants, the constituents of the culture media, the photoperiod in culture environment, stress factors, and genotype, contribute to a great extent in regulating the acquisition of somatic competence in plants (Gaj 2004). The interaction between these factors regulates somatic embryogenesis competence in plants because competent cells respond to external stimuli which trigger embryogenic pathways in plants. However, in some instances, embryogenic cells may undergo embryogenesis without external factors, a situation known as habituation possibly caused by long exposure to tissue culture environment (Pasternak et al. 2002). The following factors play significant roles in the acquisition of embryogenic competence.
Explant type
Explant type and age are part of the determining factors in the acquisition of embryogenic competence by any plant propagated through somatic embryogenesis. Hence, efforts are placed on the selection of explant parts believed to contain competent cells (cells that are capable of undergoing somatic embryogenesis stimulated by the application of external factors) (Gaj 2004). Different explant parts, like petioles, leaves, roots, and shoot meristems of some economic important plants, were reported in their propagation through somatic embryogenesis. However, the different explant parts used respond maximally at certain stages (age) of their life cycle (Castillo et al. 2000). Gaj (2004), while working on the immature leaves of Arabidopsis, indicated that the explant age influences somatic embryogenesis efficiency. Yam somatic embryogenesis is recent, at the protocol optimization stage as several researchers have worked on and are still working with different young explant parts and plant growth regulators (PGR) (Table 2).
Phytohormones and plant growth regulator (PGR) balance
Plants contain endogenous phytohormonal compounds produced in response to biotic and abiotic stresses. These hormones move within the plant and signal cellular responses to external stimuli (Opik and Rolfe 2005). On the other hand, plant growth regulators (PGRs) are synthetic compounds that act in the same way as the hormones and are classified based on their physiological mode of actions (Gutirrez-Mora et al. 2012). The endogenous phytohormone levels in plants are an important factor controlling explants’ embryogenic competence, and the quantity and quality of the phytohormones (auxins, cytokinins, and gibberellins) differ per plant explant. In Arabidopsis, an increased level of auxin expression is closely associated to cotyledon primordial, which in turn is associated with the embryogenic competence (Gaj 2004). However, information on the endogenous hormone levels of different plant genotypes at different embryogenic states is leaving no doubt as there were no significant differences in some of the hormones discovered in the competent and non-competent genotypes of sparrowgrass to somatic embryogenesis (Limanton-Grevet and Jullien 2000). Gaj (2004) has suggested that cultures responsive to callus induction, instead of displaying high endogenous hormone (auxin) levels, accumulate them, and this hormonal accumulation takes place within the first few days of the culture under embryogenic conditions in plants like carrot, alfalfa, and sunflower cells, which is a reason why researchers target the young tissues (Pasternak et al. 2002). Among the PGRs studied by different researchers for the induction of embryogenic competence pathway in plants, the auxins and cytokinins are preferred in regulating cell division in plants (Opik and Rolfe 2005). For example, changes in the auxin receptor genes altered embryogenic induction in Arabidopsis (Chen et al. 2001). Hence, the composition and concentration of auxin or cytokinin levels in plants determine both the explant’s ability to respond to morphogenic reactions and the mode of reaction (Pasternak et al. 2002).
Auxins
Thomas et al. (2002) concluded that the synthesis of auxins induced by plant explants is one of the critical signals that determine embryogenic competence in culture environments. In addition, auxins alone or in combination with cytokinin are crucial in the SE induction in plants. However, in some instances, somatic embryogenesis induction takes place in the absence of PGRs. In yam, the endogenous form of the auxin is the indole-3-acetic acid (IAA) while the synthetic form includes indole-3-butyric acid (IBA), NAA, and 2,4-D. High concentrations of NAA and 2,4-D have been used to induce callus in yam as they have proven to be active in the cellular differentiation and proliferation of plant cells (Opik and Rolfe 2005). This shows that synthetic PGRs and the auxinic herbicides, like 2,4-D, act as an effective stressor, which is a pre-requisite in callus induction in plants (Feher et al. 2003).
Cytokinins
Naturally, cytokinins occur in plants as zeatin and zeatin riboside while the synthetic form includes kinetin, thidiazuron (TDZ), benzyl adenin (BA), and BAP. The addition of these PGRs in a culture medium during somatic embryogenesis processes inhibits the induction effect of auxins (Feher 2008), although cytokinin has been used in the induction of callus in Oncidium species (Chieng et al. 2014). Nanda and Rout (2003) have also reported callus induction in a cytokinin-supplemented culture medium. However, the rate of occurrence is rare. In yam, plantlet recoveries from somatic embryos occur by the addition of BAP mostly to the culture medium. This shows that the withdrawer of auxin in callus medium and addition of cytokinin facilitates the dedifferentiation of callus to form somatic embryos (Ossai et al. 2018). However, Suarez et al. (2011) reported that the addition of BAP to a culture medium did not have significant effect on the conversion of somatic embryos to plants in the white yam genotypes evaluated.
Culture environment
Light, photoperiod, and temperature make up the in vitro environment, and their conditions affect somatic embryogenesis maximum success in plants (Gutirrez-Mora et al. 2012). The sensitivity of somatic embryogenesis developmental stages to temperature variations (freezing and non-freezing) in Catharanthus roseus (L.) has been documented (Aslem et al. 2011). In maize, rye, and rainbow pink, the application of heat shock before callus induction has considerably improved the somatic embryogenesis process, and double-fold improvement in the embryogenic response through the pre-cold treatment of maize was reported (Fu et al. 2008). However, in yam, there is a dearth of information on the influence of temperature in the somatic embryogenesis processes, which is an area to exploit in future research to improve the applicability of somatic embryogenesis in its propagation.
The importance of photoperiods on the success of somatic embryogenesis and other tissue culture techniques is invaluable (Gutirrez-Mora et al. 2012). Reports on the exploitation of photoperiods on somatic embryogenesis range from the use of phytochrome in common quince to the use of gro-lux lamps to regulate somatic embryogenesis in white bladder flowers (Torne et al. 2011). However, both light and dark conditions have shown a positive correlation with embryogenesis induction in plants (Cheong and Pooler 2004). The light intensity in a culture environment influences the efficiency and morphogenic response of explants to shoot recovery (Gaj 2004). In yam, reports on the stages of somatic embryogenesis showed that the success depends on the photoperiod of the culture environment from callus induction to embryo formation and subsequent regeneration of plantlets (Suarez et al. 2011; Ossai et al. 2018). Independent reports from the above two authors have shown that callus induction and proliferation in yam are mostly achieved in an auxin medium kept in a dark chamber while the formation of embryos and plantlet regeneration take place in a hormone-free medium and a cytokinin-supplemented medium cultured in 16-h light conditions.
Culture medium for any tissue culture system including somatic embryogenesis should contain a carbon source, macro- and micronutrients, vitamin, amino acids, growth regulators, and gelling agents (for semi-solid conditions) (Junaid et al. 2013). These constituents, except for sucrose and growth regulators, are present in the MS basal medium commonly used for different crops including yam (Suarez et al. 2011; Manahoran et al. 2016; Ossai et al. 2018). In most cases of somatic embryogenesis in yam, 30.0 g L−1 of sucrose is added in the culture medium, which serves as a carbon source to the cultures (Manahoran et al. 2016). Somatic embryogenesis has been investigated with a series of other basal media ranging from the use of Schenk and Hildebrandt (SH) (Schenk and Hildebrandt 1972) in Agave tequilana (Rodriguez-Sahagun et al. 2010) to B5 medium in Arabidopsis thaliana and N6 medium in oil palm (Thuzar et al. 2010). The optimization of these media for yam somatic embryogenesis protocol development is essential, as not all will be readily available at different geographical locations in a given time.
Stress factors
It is now widely established that somatic cells or somatic viable plant explants acquire embryogenic competence as a result of the explants’ interaction with the chemical and physical factors present in the culture medium, which are generally known as stress conditions, as the level of these factors deviates from normal (Feher et al. 2003). Stress is a pre-requisite to the induction of embryogenic competence in plants, and this comes as a natural way of plant response to external stimuli. These stresses can be advantageous in manipulating the cultures as osmotic pressure, wounds, temperature extremes, and starvation, as the imbalance they create can trigger a plasticity response in plants similar to a defense mechanism thereby causing cellular reprogramming and dedifferentiation (Feher 2008).
Genotype
Genotype is one of the major determining factors in the growth of cultured organs and morphogenesis in vitro (Junaid et al. 2013). Sharma (2008) reported the genotypic control of somatic embryogenesis in potato. In melon, research has shown that inodurus variety responds poorly to somatic embryogenesis as compared to the reticulatus (Stipp et al. 2001). In Asparagus officinalis L., the genotype by explant interaction was significant for callus induction while there was also a positive relationship between barley genotypes and the culture medium for callus induction (Hanzel et al. 1985). Somatic embryogenesis research in yam has shown variable degrees of genotype-dependent response. In research conducted by Ossai et al. (2018) on the development of an optimum system for yam micropropagation using somatic embryogenesis, there were variations in the genotypic response to the somatic embryogenesis processes between and within the species. Genotypic response in yam is so variable that it has restricted progress in the full applicability of somatic embryogenesis (Manoharan et al. 2016). In this regard, it was clear that the differences seen in the response between varieties are genetically controlled (George 1993).
Genetic control of somatic embryogenesis
Molecular studies of somatic embryogenesis in Arabidopsis have shown that auxin level in an explant controls chromatin remodeling and expressed genes at the induction phase of somatic embryos as high levels of auxin bring about the demethylation of deoxyribonucleic acid in dividing cells which is a requirement for pro-embryo formation (Fehér 2008). Thus, the exogenous application of auxin in the culture medium helps in determining embryogenic acquisition (Thomas and Jiménez 2005). However, plants’ endogenous hormone appears inadequate for constructing a marker for somatic embryogenesis studies as Feher et al. (2003) suggested that the somatic embryo induction phase is more of a stress response. Due to the above reasons, other factors help in determining the somatic embryogenesis competence of explants (Thomas and Jimenez 2005). Hence, cell differentiation during embryogenic acquisition is controlled by the expression of genes in plants (Feher et al. 2003). Komamine et al. (2005) have described these expressed genes during somatic embryogenesis processes as being phase specific. This has thus necessitated gene expression studies in somatic embryos while using both embryogenic and non-embryogenic tissues (Chung and Karana 2002) with some of the expressed genes (Table 3) discussed below.
WUSCHEL (WUS)
The expression of this gene is cytokinin dependent with an increased expression level a few days after explant excision as observed in Medicago truncatula and Arabidopsis (Chen et al. 2009). This validates the work of Gordon et al. (2007) who had earlier reported that cytokinin induces shoots and WUS expression in Arabidopsis, and that the early WUS expression in plants is a characteristic of somatic embryo induction. In the zygotic embryogenesis process of Arabidopsis, the detection of WUS gene was at the shoot apical meristem stem cell niche, which precedes bipolar embryo formation in plants (Chen et al. 2003). This suggests that WUS initiates the embryogenic stem cells that progress into embryogenesis (Rose 2019) and its inclusion in the transformation process of hitherto recalcitrant maize line has resulted to a success rate of up to 40% embryogenic recoveries (Lowe et al. 2016; Hoerster et al. 2020).
Somatic Embryo Related Factor 1 (SERF1) gene
This gene was discovered between callus and somatic embryo induction of barrelclover (Mantiri et al. 2008). From the study, the expression of this gene is upregulated at 21 d of culture which falls between callus and somatic embryo development, although it requires an ethylene, cytokinin, and auxin presence in plants (Rose 2019). Since this gene has the WUS binding sites, it is suggested that its expression is related to the WUS gene, and it can still be detected up to the heart stage of embryo development (Mantiri et al. 2008).
Baby Boom (BBM)
This gene was discovered at the early stage of SE in A. thaliana and rape, and its expression at the heart-shaped stage of somatic embryos is said to enhance somatic embryo formation in cocoa (Kulinska-Lukaszek et al. 2012). This gene triggers other genes like LEAFY COTYLEDON (LEC1), ABA INSENSITIVE (ABI3), and FUSCA3 (FUS3) to activate SE (Horstman et al. 2017) in plants. Boutilier et al. (2002) also suggested that it promotes SE in rape.
LEAFY COTYLEDON (Lec1)
The overexpression of LEAFY COTYLEDON (Lec1) gene of Arabidopsis was directly linked to the induction of embryo development on vegetative tissues even without the exogenous application of auxins in the culture environment and the Lec1 gene is expressed until the late torpedo stage of embryo development (Feher et al. 2003). Similar to the lec1 gene in function is the lec2 gene, which was also identified in Arabidopsis, and it is required by plants at both early and late stages of embryo development as they confer embryonic characters to plant tissues (Stone et al. 2001).
Somatic Embryogenesis Receptor Kinase (SERK) gene
The induction of embryogenic cells from somatic cells is characterized by RNA and DNA synthesis and elevated enzyme activities, mainly the kinase group (Suprasanna and Bapat 2005). Dhananjay and Bhupendra (2014) were able to show that SE receptor kinase (SERK1) gene level was responsible for the differences between the frequently regenerating lines and the non-regenerating lines of cotton via SE. This gene has been earlier reported to be the only known gene that directs embryogenesis in plant cells (Dhananjay and Bhupendra 2014). The SERK gene encodes for an N-terminal domain protein, leucine-rich repeats, and the proline-rich region, which is a conserved feature of extension (Dhananjay and Bhupendra 2014). This SERK gene has been detected at the somatic embryo formation stage of carrot (Shah et al. 2001) while seedling-derived callus of carrot overexpressing AtSERK1 gene had a three to four times higher embryogenic competence than its wild type–derived callus (Suprasanna and Bapat 2005). However, the SERK gene is the only gene that controls the acquisition of embryogenic competence in different plants but ceases to be expressed after the globular stage of embryo formation (Feher et al. 2003).
Somatic embryogenesis and biotechnology application in yam
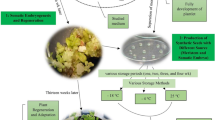
Biotechnology is simply a technique that utilizes an organism to modify a product with the aim of improving the product for specific uses relative to its initial status (Persley 1992). Brink et al. (1998) identified three main areas of biotechnology application: plant molecular marker identification and construction, genetic engineering, and plant tissue culture (organogenesis and somatic embryogenesis). Apart from the improved propagation ratio of yam achieved through optimized somatic embryogenesis protocols (Ossai et al. 2018), the SE system offers several opportunities for the biotechnology application, such as cryopreservation, somatic hybridization, and genetic transformation (Manoharan et al. 2016). This will play a direct role in boosting food production in Africa through the availability of clean seed yam to farmers all year-round. Brink et al. (1998) stressed that the application of biotechnology in Africa can be achieved in three main phases to boost vegetative propagated crops (VPCs). These phases are the following: first, the optimization of in vitro propagation methods to rapidly multiply disease-free plants and the creation of a regenerative system for plant transformation. Manoharan et al. (2016), Ossai et al. (2018), and Suarez et al. (2011) have extensively worked on optimizing this regenerative phase for yam.
The second phase involves the application of biotechnological tools to improve selection efficiencies of improved varieties, such as anther culture, embryo rescue, and marker-assisted breeding. The term “anther culture’’ is a process that produces haploid embryos, using immature pollens in anthers cultivated on nutrition media (Hosseini et al. 2015). According to Barakat et al. (2012), this system requires a short time period to conduct and could accelerate the production of improved traits, and it has been reported in over 200 species of angiosperms (Hosseini et al. 2015). Factors, such as the genotype of the donor plant, the stage of pollen development, composition of the nutrient medium, pretreatment of flower buds, and the physiological state and conditions of growth of donor plants, affect the induction of androgenesis (Smykal 2000).
Ovule culture
This is a process of haploid regeneration via unpollinated female gametophytes (gynogenesis) (Jin-Feng et al. 2010). It is also a possible source of haploid production in plants especially in recalcitrant plants due to male sterility and the dioecious nature of plants (Bhat and Murthy 2007). Successful haploid production from unfertilized ovules was reported in potato (Ruth et al. 1993), maize (Tang et al. 2006), cucumber (Gemenes-Juhasz et al. 2002), and yam (Himanshu et al. 2016).
Protoplast fusion
Plant somatic hybridization via protoplast fusion has become an important tool for ploidy manipulation in plant improvement schemes, allowing researchers to combine somatic cells from different cultivars, species, or genera, resulting in novel allotetraploid and autotetraploid genetic combinations (Jude et al. 2011). This technique can facilitate conventional breeding, gene transfer, and cultivar development by bypassing some problems associated with conventional sexual hybridization, including sexual incompatibility, nucellar embryogenesis, and male or female sterility (Wang et al. 2022). The most common target using somatic hybridization is the generation of symmetric allotetraploid hybrids that contain the complete nuclear genomes of both parents (Jude et al. 2011). The polyethylene glycol (PEG)-mediated method is one of the most successful techniques for protoplast fusion that attempts to enhance agglutination to produce intergeneric somatic hybrids. A PEG-mediated method has been used extensively for its simplicity, efficiency, and economy, and does not seem to interfere with protoplast viability (Grosser and Gmitter 2011). However, protoplast fusion requires the establishment of an efficient system of protoplast isolation followed by cell division and plant regeneration (Nassour and Dorion 2002). Despite the success recorded in citrus (Grosser and Gmitter 2005), rice (Jude et al. 2011), tomato, and potato (Orczyk et al. 2003), there has been little or no report on its applicability in yam.
Cryopreservation
The last but not the least factor on the utilization of SE in the improvement of crops in Africa through biotechnology is cryopreservation. This involves the storage of somatic embryos and other plant materials at low temperature (–196 °C) usually achieved through the use of liquid nitrogen for safe, cost-effective, and long-term conservation of yam embryos for future manipulation and use. While somatic embryos have been successfully produced in yam, there are currently no information on the use of cryopreservation in conserving yam somatic embryos unlike other VPCs like potato (Bhatti et al. 1997), banana (Sonali 2005; Li et al. 2010), and cassava (Stewart et al. 2001). However, since it has been done in other VPCs, it will be feasible for yam as a protocol for cryopreserving other yam explants, like meristem, axillary buds, and stem, and has been reported (Mandal and Sonali 2007).
The third phase is to develop operational capacity for the production of transgenic plants, gene isolation, and cloning and gene insertion. A recent advancement in the development of transgenic plants involves the utilization of technologies like the CRISPR/Cas-based genome editing (Wang et al. 2022). This technology has been deployed in the modification of VPCs, like potato and banana, although that of banana is still at the early-stage deployment in the improvement of banana nutrient content and disease resistance (Tripathi et al. 2019; Tripathi et al. 2022). However, this modification technology has not been deployed in the genetic improvement of yam that really needed it due to the numerous challenges facing conventional yam breeding, like the non-synchronization of flowering that hinders, poor understanding of genetic diversity, and limited enabling techniques for breeding (Mondo et al. 2020).
Conclusion
Availability of quality and affordable seed yam is key in the actualization of food security in Africa where it is an integral part of the culture as it has been nicknamed king of crops. This review has shown the trend of yam propagation from the informal seed system (traditional methods) characterized with a low propagation ratio to the formal seed system with valuable improvement in the propagation ratio with additional means of cleaning infected ones. Somatic embryogenesis, when fully optimized, offers a faster multiplication rate with a clear pathway for genetic engineering, germplasm conservation, and exchange through synthetic seeds. However, unlike other plants, the applicability of SE in yam propagation is still at an early stage and is constrained by the low callus induction rate and genotype dependence, which are areas for future research to understand the molecular and epigenetic basis as much of the work done is on the optimization and development of culture protocols. The full knowledge of SE regeneration pathway is a pre-requisite for the efficient deployment of other biotechnological techniques in boosting yam production in Africa.
Data availability
Not applicable.
References
Agbele SO, Ayankanmi TG, Kikuno H (2010) Effects of synthetic hormone substitutes and genotypes on rooting and mini tuber production of vines cuttings obtained from white yam (Dioscorea rotundata, Poir). African J Biotechnol 9:4714–4724
Aighewi BA, Asiedu R, Maroya N, Balogun M (2015) Improved propagation methods to raise the productivity of yam (Dioscorea rotundata Poir.). Food Secur 7:823–834
Anil VS, Harmon AC, Sankara Rao K (2000) Spatio-temporal accumulation and activity of calcium-dependent protein kinases during embryogenesis, seed development, and germination in sandalwood. Plant Physiol 122:1035–1043
Aslam J, Mujib A, Sharma MP (2011) Influence of freezing and non-freezing temperature on somatic embryogenesis and vinblastine production in Catharanthus roseus (L.) G. Don Acta Physiol Plant 33:473–480
Balestrazzi A, Toscano I, Bernacchia G, Luo M, Otte S, Carbonera D (1996) Cloning of a cDNA encoding DNA topoisomerase I in Daucus carota and expression analysis in relation to proliferation. Gene 183:183–190
Balogun MO, Maroya N, Asiedu R (2014) Status and prospects for improving yam seed systems using temporary immersion bioreactors. Academic J 13:1614–1622
Balogun MO, Maroya N, Ossai C, Ajayi A, Aighewi B, Asiedu R (2018) Breeder seed yam production from soil to soilless systems: yam hydroponics. pp 4–25
Barakat MN, Al-doss AA, Elshafei AA, Moustafa KA, Ahmed EI (2012) Anther culture response in wheat (Triticum aestivum L.) genotypes with HMW alleles. Cereal Res Comm 40:583–591
Belarmino M, Gonzales J (2008) Somatic embryogenesis and plant regeneration in purple food yam (Dioscorea alata L.). Annals of Tropical Research 30:22–33
Bhat JG, Murthy HN (2007) Factors affecting in vitro gynogenic haploid production in Niger (Guizotia abyssinica (L.F.) Cass.). Plant Grow Regul 52:241–248
Bhatti MH, Percival T, Davey CDM, Henshaw GG, Blackesley D (1997) Cryopreservation of embryogenic tissue of a range of genotypes of sweet potato (Ipomoea batatas [L] Lam.) using an encapsulation protocol. Plant Cell Rep 16:802–806
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JB, van LookerenCampagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Brink JA, Woodward BR, DaSilva E (1998) Plant biotechnology: a tool for development in Africa. EJB Electronic J Biotechnol 1:1–10
Castillo P, Marquez J, Rubluo A, Hernandez G, Lara M (2000) Plant regeneration from callus and suspension cultures of Valeriana edulis ssp. Procera via simultaneous organogenesis and somatic embryogenesis. Plant Sci 151:115–119
Chen JT, Chang WC (2001) Effects of auxins and cytokinins on direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramsey.’ Plant Grow Regul 34:229–232
Chen Y, Fan J, Yi F, Luo Z, Fu Y (2003) Rapid clonal propagation of Dioscorea zingiberensis. Plant Cell Tiss Org Cult 73:75–80
Cheong EJ, Pooler MR (2004) Factors affecting somatic embryogenesis in Prunus incisa cv. February Pink Plant Cell Rep 22:810–815
Chen SK, Kurdyukov S, Kereszt A, Wang XD, Gresshoff PM, Rose RJ (2009) The association of homeobox gene expression with stem cell formation and morphogenesis in cultured. M truncatula 230:827–840
Chieng LMN, Chen TY, Sim SL, Goh DKS (2014) Induction of organogenesis and somatic embryogenesis of Gonystylus bancanus (Miq.) Kurz (Ramin). Sarawak Forestry Corporation and ITTO. Pp 1
Chung A, Khurana P (2002) Gene expression during somatic embryogenesis; recent advances. Curr Sci 86:715–730
Curaba J, Moritz T, Blervaque R, Parcy F, Raz V, Herzog M, Vachon G (2004) AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol 36:3660–3669
Dhananjay KP, Bhupendra C (2014) Role of plant somatic embryogenesis receptor kinases (SERKs) in cell-to-embryo transitional activity: key at novel assorted structural subunits”. Amer J Plant Sci 5:3177–3193
Elhiti M, Stasolla C, Wang A (2013) Molecular regulation of plant somatic embryogenesis. In Vitro Cell Dev Biol – Plant 49:631–642
Fehér A (2008) The initiation phase of somatic embryogenesis: what we know and what we don’t. Acta Biol Szegediensis 52:53–56
Fehér A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org Cult 74:201–228
Fu XP, Yang SH, Bao MZ (2008) Factors affecting somatic embryogenesis in anther cultures of Chinese pink (Dianthus chinensis L.). In Vitro Cell Dev Biol - Plant 44:194–202
Gaj MD (2004) Factors influencing somatic embryogenesis and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Cell Tiss Org Cult 43:27–27
Gemenes-Juhasz A, Balogh P, Ferenczy A, Kristof Z (2002) Effects of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Reprod 25:105–111
George EF (1993) Plant propagation by tissue culture. Part 1. Technol Plant Biosys 135:79–84
Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM (2007) Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134:3539–3548
Grosser JW, Gmitter FG Jr (2005) 2004 SIVB Congress symposium proceedings thinking outside the cell— applications of somatic hybridization and cybridization in crop improvement, with citrus as a model. In Vitro Cell Dev Plant 41:220–225
Grosser JW, Gmitter FG Jr (2011) Protoplast fusion for production of tetraploids and triploids: applications for scion and rootstock breeding in citrus. Plant Cell Tiss Org Cult 104:343–357
Gulzar B, Mujib A, Malik MQ, Sayeed R, Mamgain J, Ejaz B (2020) Genes, proteins and other networks regulating somatic embryogenesis in plants. J Genet Eng Biotechnol 18:31. https://doi.org/10.1186/s43141-020-00047-5.PMID:32661633;PMCID:PMC7359197
Gutiérrez-Mora A, González-Gutiérrez A, Rodriguez-Garay B, Ascencio-Cabral A, Li-Wei L (2012) Plant somatic embryogenesis: some useful considerations. Embryogenesis, Dr. Ken-Ichi Sato (Ed.), ISBN: 978–953–51–0466–7, InTech, pp. 1–22. Available from: http://www.intechopen.com/books/embryogenesis/somatic-embryogenesis-some-useful-considerations
Hagen G, Kleinschmidt A, Guilfoyle T (1984) Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 162:147–153
Hanzel JJ, Miller JP, Brinkman MA, Fendos E (1985) Genotype and media effects on callus formation and regeneration in barley. Crop Sci 25:27–31
Higashi K, Shiota H, Kamada H (1998) Patterns of expression of the genes for glutamine synthetase isoforms during somatic and zygotic embryogenesis in carrot. Plant Cell Physiol 39:418–424
Hoerster G, Wang N, Ryan L, Wu E, Anand A, McBride K, Lowe K, Jones T, Gordon-Kamm B (2020) Use of non-integrating Zm-Wus2 vectors to enhance maize transformation. In Vitro Cell Dev Biol - Plant 56:265–279
Horstman A, Li M, Heidmann I, Weemen M, Chen B, Muiño JM (2017) The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol 175:848–857
Hosseini SZ, Mohammadi PP, Cheshmeali GR (2015) Effect of induction medium on embryogenesis of maize anther culture and increase of normal haploid plantlet regeneration with Wathman filter paper. Iran J Plant Physiol 5:1503–1510
Ikeda M, Umehara M, Kamada H (2006) Embryogenesis-related genes; its expression and roles during somatic and zygotic embryogenesis in carrot and Arabidopsis. Plant Biotechnol 23:153–161
Jin-Fen C, Li C, Ahmed AM, Kere GM (2010) In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tiss Org Cult 104:311–319. https://doi.org/10.1007/s11240-010-9874-6
Jude W, Grosser JW, Gmitter FG (2011) Protoplast fusion for production of tetraploids and triploids: applications for scion and rootstock breeding in citrus. Plant Cell Tiss Org Cult 104:343–357
Junaid A, Srivastava PS, Sharma MP (2013) Chapter: Factors regulating somatic embryogenesis in plants. Somatic embryogenesis and gene expression, Edition: 1st Narosa Publishing House, New Delhi, pp 56:81
Kagaya Y, Toyoshima R, Okuda R, Usui H, Yamamoto A, Hattori T (2005) LEAFY COTYLEDON1 controls seed storage protein genes through its regulation of FUSCA3 and ABSCISIC ACID INSENSITIVE3. Plant Cell Physiol 46:399–406
Komamine A, Murata N, Nomura K (2005) 2004 SIVB Congress Symposium Proceeding: Mechanisms of somatic embryogenesis in carrot suspension cultures. Morphology, physiology, biochemistry, and molecular biology. In Vitro Cell Dev Biol - Plant 41:6–10
Kulinska-Lukaszek K, Tobojka M, Adamiok A, Kurczynska E (2012) Expression of the BBM gene during somatic embryogenesis of Arabidopsis thaliana . Biol Plant 56:389–394
Li Y, Wei Y, Hu G, Chen H, Xu C (2010) Plant regeneration via somatic embryogenesis after cryopreservation of embryogenic cell suspensions of banana (Musa spp. AAA) by vitrification and the genetic stability of regenerated plant. Acta Hort Sinica 37:899–905
Limanton-Grevet A, Jullien M (2000) Somatic embryogenesis in Asparagus officinalis can be an in vitro selection process leading to habituated and 2,4-D dependent embryogenic lines. Plant Physiol Biochem 38:567–576
Liu HI, Wang GC, Feng Z, Zhu J (2010) Screening of genes associated with dedifferentiation and effect of LBD29 on pericycle cells in Arabidopsis thaliana. Plant Growth Reg 62:127–136
Lowe K, Emily W, Ning W, George H, Craig H, Myeong-Je C, Chris S, Brian L, Mark C, Josh C, Lijuan W, Larisa R, Tanveer K, Julia C, Wei H, Maryanne Y, Jenny B, Zhongmeng B, Kent B, Elizabeth I, Bhojaraja R, Shamseer PM, Wes B, Lisa N, Bo S, Peizhong Z, Dennis B, Carl F, Jim R, Zuo-Yu Z, Deping X, Todd J, William G (2016) Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28:1998–2015. https://doi.org/10.1105/tpc.16.00124
Maheswari RU, Prabha AL, Nandagopalam VN, Amburaja V (2012) In vitro rhizome production from nodal explants and callus formation of the medicinal plant (D. oppositifolia L.). IORS J Pharm Biol Sci 1:17–21
Mandal B, Sonali M (2007) Cryopreservation of in vitro shoot tips of Dioscorea deltoidea Wall., an endangered medicinal plant: effect of cryogenic procedure and storage duration. Cryo Letters 28:461–470
Manoharan R, Tripathi JN, Tripathi L (2016) Plant regeneration from axillary bud derived callus in white yam (Dioscorea rotundata). Plant Cell Tiss Org Cult 126:481–497
Mantiri FR, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang XD (2008) The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in M. truncatula . Plant Physiol 146:1622–1636
Maroya N, Balogun M, Aighewi B, Kumar PL, Ogbole S, Asiedu R (2017) Seed yam production using single node vine from plants in aeroponics. IITA, Ibadan, Nigeria. pp 1–4
Maroya N, Balogun M, Asiedu R, Aighewi B, Kumar PL, Augusto J (2014) Yam propagation using aeroponics technology. Ann Res Rev Biol 4:3894–3903
Mondo JM, Agre PA, Edemodu A, Adebola P, Asiedu R, Akoroda MO, Asfaw A (2020) Floral biology and pollination efficiency in yam (Dioscorea spp.). Agriculture 10:560–585. https://doi.org/10.3390/agriculture10110560
Nanda R, Rout G (2003) In vitro somatic embryogenesis and plant regeneration in Acacia Arabica. Plant Cell Tiss and Org Cult 73:131–135
Nassour M, Dorion N (2002) Plant regeneration from protoplasts of micropropagated Pelargonium x hurtorum “Alain”: effect of some environmental and medium factors on protoplast system efficiency. Plant Sci 163:169–176
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 133:218–230
Öpik H, Rolfe S (2005) Plant growth hormones In: The physiology of flowering plants. 4th edition. Willis A. J. pp177 – 202
Orczyk W, Przetakiewicz J, Nadolska-Orczyk A (2003) Somatic hybrids of Solanum tuberosum: application to genetics and breeding. Plant Cell Tiss Org Cult 73:245–256
Ossai C, Balogun M, Maroya N Asiedu R (2018) Development of protocols for somatic embryogenesis in yam (Dioscorea spp.) towards scale up production in temporary immersion bioreactor system. YIIFSWA Research Brief pp. 1–7
Otoo E, Anyakanmi TG, Kikuno H, Asiedu R (2016) In vivo yam (Dioscorea spp.) vine multiplication technique: the plausible solution to seed yam generation menace. J Agr Sci 8:88–97
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen HA, Dudits D, Feher A (2002) The role of auxins, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol 129:1807–1819
Persley GJ (1992) Beyond Mendel’s garden: biotechnology in agriculture. In: Biotechnology enhancing research on tropical crops in Africa. CTA/IITA co-publication, p 11–19
Rodríguez-Sahagún A, Acevedo-Hernández G, Rodríguez-Domínguez JM, Rodríguez-Garay B, Cervantes-Martínez J, Castellanos-Hernández OA (2010) Effect of light quality and culture medium on somatic embryogenesis of Agave tequilana Weber var. Azul Plant Cell Tiss Org Cult 104:271–275
Rose RJ (2019) Somatic embryogenesis in the Medicago truncatula model: cellular and molecular mechanisms. Front Plant Sci 10:1–14
Ruth SK, Stephen LS, John CB (1993) Ovule culture of sweet potato (Ipomea batatas) and closely related species. Plant Cell Tiss Org Cult 32:77–82
Saini H, Kashihara Y, Lopez-Montes A, Asiedu R (2016) Interspecific crossing between yam species (Dioscorea rotundata and Dioscorea bulbifera) through in vitro ovule culture. American J Plant Sci 7:1268–1274. https://doi.org/10.4236/ajps.2016.78122
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian J Bot 50:199–204. https://doi.org/10.1139/b72-026
Shah K, Schmidt EDL, Vlak JM, de Vries SC (2001) Expression of the Daucus carota somatic embryogenesis receptor kinase (DcSERK) protein in insect cells. Biochimie 83:415–421
Sharma KS, Millam S, Bryan HI, GJ, (2008) Cloning and molecular characterization of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta 228:319–330
Shu Y, Ying-Cai Y, Hong-Hui L (2005) Plant regeneration through somatic embryogenesis from callus cultures of Dioscorea zingiberensis. Plant Cell Tiss Org Cult 80:157–161
Smykal P (2000) Pollen embryodenesis: the stress mediated switch from gametophytic to sporophytic development; current status and future prospects. Biol Plant 43:481–489
Sonali DS (2005) Cryopreservation of somatic embryos – an overview. Indian J Biotech pp 4:47–55
Stewart P, Taylor M, Mycock D (2001) The sequence of the preparative procedures affects the cryostorage of cassava somatic embryos. Cryo Lett 22:35–42
Stipp LCL, Mendes BMJ, Piedade SMD, Rodriguez APM (2001) In vitro morphogenesis of Cucumis melo var. inodorus. Plant Cell Tiss Org Cult 65:81–89
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98:11806–11811
Suarez PIE, Torres ALA, Litz R (2011) Somatic embryogenesis in yam (Dioscorea rotundata). The Revista Facultad Nacional De Agronomía Medellín 64:6037–6042
Suprasanna P, Bapat V A (2005) Differential gene expression during somatic embryogenesis. In: Mujib, A. and Samaj, J. eds. Somatic embryogenesis, Plant Cell Monographs. Berlin; Springer-Verlag 2:305–320
Tang F, Tao Y, Wang ZT, G, (2006) In vitro production of haploid and double haploid plants from pollinated ovaries of maize (Zea mays). Plant Cell Tiss Org Cult 84:233–237
Thomas C, Jiménez VM (2005) Mode of action of plant hormones and plant growth regulators during induction of somatic embryogenesis: molecular aspects. In: Mujib A, Samaj J (eds) Somatic embryogenesis, Plant Cell Monographs. Berlin; Springer- Verlag 2:157–175
Thomas C, Meyer D, Himber C, Steinmetz A (2002) Spatial expression of sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol Biochem 42:35–42
Thuzar M, Vanavichit A, Tragoonrung S, Jantasuriyarat C (2010) Efficient and rapid plant regeneration of oil palm zygotic embryos cv. “Tenera” through somatic embryogenesis. Acta Physiol Plant 33:123–128
Torné JM, Moysset L, Santos M, Simón E (2011) Effects of light quality on somatic embryogenesis in Araujia sericifera. Physiol Plant 111:405–411
Tripathi JN, Ntui VO, Ron M (2019) CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun Biol 2:46. https://doi.org/10.1038/s42003-019-0288-7
Tripathi L, Ntui VO, Tripathi JN (2022) Control of bacterial diseases of banana using CRISPR/Cas-based gene editing. Int J Mol Sci 23:3619
Wang J, Gan S, Zheng Y, Jin Z, Cheng Y, Liu J (2022) Banana somatic embryogenesis and biotechnological application. Tropic Plant 1:12. https://doi.org/10.48130/TP-2022-0012
Yang H, Saitou T, Komeda Y, Harada H, Kamada H (1997) Arabidopsis thaliana ECP63 encoding a LEA protein is located in chromosome 4. Gene 184:83–88
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
We acknowledge the support of Bill and Melinda Gates Foundation through the YIIFSWA II project of IITA, Ibadan.
Funding
This review is part of PhD work supported by the Bill and Melinda Gates Foundation (OPP1159088).
Author information
Authors and Affiliations
Contributions
Conceptualization: COO, MOB, and NGM. Data curation: COO. Formal analysis: COO. Writing–original draft: COO. Writing–review and editing: COO, MOB, and NGM.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ossai, C.O., Balogun, M.O. & Maroya, N.G. Status and prospects of yam somatic embryogenesis: a pathway for biotechnology applications. In Vitro Cell.Dev.Biol.-Plant (2024). https://doi.org/10.1007/s11627-024-10413-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11627-024-10413-4