Abstract
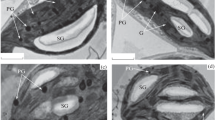
Carbon is essential for plant growth. Starch, the main storage carbohydrate, plays an important role in the plant life cycle. When poplar cells cultured normally at 25°C were subjected to low-temperature stress (at 4°C), their growth rate was dramatically inhibited. The growth rate was restored by transferring those cells to culture at room temperature. The effects of low-temperature stress on intracellular starch granules were also analyzed using an electron microscope. Under low-temperature conditions, starch within plastids rapidly decreased. As with the growth rate, when the growth environment was restored to the normal culture temperature (25°C), starch degradation in the cells was immediately suppressed and starch granules were newly synthesized in the plastids. The amount of starch in the cells was maintained at a constant level under normal conditions. The starch levels also rapidly decreased at low temperatures, as shown by their cellular aspects. Changes in the soluble sugar content of cells subjected to low temperature were determined. There was little change in the contents of glucose, sucrose, or fructose with cold stress. Only maltose showed a noticeable increase under low-temperature conditions. In summary, the results indicate that low-temperature stress induces starch degradation in poplar cells, resulting in increased maltose content.




Similar content being viewed by others
References
Bollmark L, Sennerby-Forsse L, Ericsson T (1999) Seasonal dynamics and effects of nitrogen supply rate on nitrogen and carbohydrate reserves in cutting-derived Salix viminalis plants. Can J for Res 29:85–94
Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Biol 40:305–346
Gray GR, Heath D (2005) A global reorganization of the metabolome in Arabidopsis during cold acclimation is revealed by metabolic fingerprinting. Physiol Plant 124:236–248
Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK (2006) Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 142:98–112
Johansson T (1993) Seasonal changes in contents of root starch and soluble carbohydrates in 4–6-year old Betula pubescens and Popuius tremula. Scand J Forest Res 8:94–106
Jordy MN (2004) Seasonal variation of organogenetic activity and reserves allocation in the shoot apex of Pinus pinaster Ait. Ann Bot 93:25–37
Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 136:4159–4168
Kasuga J, Arakawa K, Fujikawa S (2007) High accumulation of soluble sugars in deep supercooling Japanese white birch xylem parenchyma cells. New Phytol 174:569–579
Landhäusser SM, Lieffers VJ (2003) Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees Struct Funct 17:471–476
Lu Y, Sharkey TD (2006) The importance of maltose in transitory starch breakdown. Plant Cell Environ 29:353–366
Lustinec J, Hadacová V, Kamínek M, Procházka Z (1983) Quantitative determination of starch, amylose, and amylopectin in plant tissues using glass fiber paper. Anal Biochem 132:265–271
Maki H, Sato M, Ogawa K, Kaida R, Yamamoto N, Kaneko T (2011) Cloning and expression profile of ERF gene isolated from cold-stressed poplar cells (Populus nigra). Cytologia 76:11–18
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303:87–89
Regier N, Streb S, Zeeman SC, Frey B (2010) Seasonal changes in starch and sugar content of poplar (Populus deltoides x nigra cv. Dorskamp) and the impact of stem girdling on carbohydrate allocation to roots. Tree Physiol 30:979–987
Rohde P, Hincha DK, Heyer AG (2004) Heterosis in the freezing tolerance of crosses between two Arabidopsis thaliana accessions (Columbia-0 and C24) that show differences in non-acclimated and acclimated freezing tolerance. Plant J 38:790–799
Sagisaka S (1992) A cold environment is a perquisite for formation of “plastid initials” in winter buds of poplar. Plant Physiol 99:1657–1663
Sauter JJ, van Cleve B (1991) Biochemical, immunochemical, and ultrastructural studies of protein storage in poplar (Populus × Canadensis ‘robust’) wood. Planta 183:92–100
Seung D, Schreier TB, Bürgy L, Eicke S, Zeeman SC (2018) Two plastidial coiled-coil proteins are essential for normal starch granule initiation in Arabidopsis. Plant Cell 30:1523–1542
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, Von Korff M, Steinhauser MC, Keurentjes JJ, Guenther M, Hoehne M, Selbig J, Fernie AR, Altmann T, Stitt M (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106:10348–10353
Suzuki H, Kato E, Matsuzaki A, Ishikawa M, Harada Y, Tanikawa K, Nakagawa H (2009) Analysis of saccharides possessing post-translational protein modifications by phenylhydrazine labeling using high-performance liquid chromatography. Anal Sci 25:1039–1042
Wiemken V, Ineichen K (1993) Effect of temperature and photoperiod on the raffinose content of spruce roots. Planta 190:387–392
Yu TS, Zeeman SC, Thorneycroft D, Fulton DC, Dunstan H, Lue WL, Hegemann B, Tung SY, Umemoto T, Chapple A, Tsai DL, Wang SM, Smith AM, Chen J, Smith SM (2005) Alpha-amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J Biol Chem 18:9773–9779
Zhao L, Yang T, Xing C, Dong H, Qi K, Gao J, Tao S, Wu J, Wu J, Zhang S, Huang X (2019) The beta-amylase PbrBAM3 from pear (Pyrus betulaefolia) regulates soluble sugar accumulation and ROS homeostasis in response to cold stress. Plant Sci 287:110184
Zhu H, Yang X, Wang X, Li Q, Guo J, Ma T, Zhao C, Tang Y, Qiao L, Wang J, Sui J (2021) The sweetpotato beta-amylase gene IbBAM1.1 enhances drought and salt stress resistance by regulating ROS homeostasis and osmotic balance. Plant Physiol Biochem 168:167–176
Acknowledgements
The author thanks Dr. Mamiko Sato (Laboratory of Electron Microscope, Japan Women’s University) for the support in using electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Rights and permissions
About this article
Cite this article
Maki, H. Degradation of starch in poplar cells at low temperatures. In Vitro Cell.Dev.Biol.-Plant 58, 781–786 (2022). https://doi.org/10.1007/s11627-022-10282-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-022-10282-9