Abstract
Induction of androgenesis, followed by chromosome doubling, is a crucial method to obtain complete homozygosity in one-generation route. However, in vitro androgenesis can result in various genetic and epigenetic changes in derived triticale plants. In this study, we evaluated chromosome alternations and we associated them with the changes of spike morphology in androgenic progeny of triticale. We karyotyped offspring plants that derived from double haploid plants using fluorescence in situ hybridization techniques. We distinguished four major groups of karyotypes: double ditelosomics, nullisomics N2R, nullisomics N5R, and triticale plants with a complete set of chromosomes. It is known that more than half of QTLs connected with androgenic response are located in R-genome of triticale but 2R, 5R, and 6R chromosomes are not included. We hypothesized that the reason why only aberrations of chromosomes 2R and 5R appear during androgenesis of triticale is that because these chromosomes are not involved in the stimulation of androgenic response and the following regeneration of plants is not disrupted. Concerning the established groups, we evaluated following quantitative traits: spike length, number of spikes per plant, number of spikelets per spike, and number of grains per spike. The nullisomy of chromosome 2R and 5R resulted in vast changes in spike architecture of triticale plants, which can be correlated with the location of major QTLs for spike morphology traits on these chromosomes. The spikes of nullisomic plants had significantly decreased spike length which correlated with the reduction of number of spikelets per spike and number of grains per spike.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triticale (× Triticosecale Wittmack) is an example of human-made polyploid, which was produced through crossing between wheat (Triticum aestivum L.) and rye (Secale cereale L.). First triticale was produced by Wilson (1876), which launches the efforts to develop a new crop, that consolidate the quality properties of wheat with the resistance to environmental stresses of rye. Over the last 40 yr, triticale has gained considerable importance with the worldwide harvested area increased from 467 ha in 1975 to 4,165,783 ha in 2017 (FAO 2016). The main cultivation area is located in the European Union (2,773,681 ha; FAO 2016).
Double haploid production is one of the most important improvements in modern breeding methods (Thomas et al.2003; Oettler et al.2005). It assures the one-generation route to homozygosity, which saves time, comparing to classical 5–7-yr-long pedigree programs (Dwivedi et al.2015; Weigt et al.2016; Weigt et al.2017). However, the development of new cultivar can last 8 to 12 yr, including registration tests (Wiśniewska et al.2019). Lots of triticale-specific modifications have been invented and exploited in practice by geneticists and breeders. Protocols used for DH production in triticale have been developing based on wheat protocols (Eudes and Chugh 2009). Androgenesis in triticale is performed both by the anther culture (Ślusarkiewicz-Jarzina et al.2017) and with the microspore culture methods (Pauk et al.2000). However, the triticale anther culture is routinely used by breeding companies, because of low costs and easier protocols to handle in comparison with microspore culture. Recently, the development of triticale DH lines is a standard procedure in breeding programs (Tuvesson et al.2003).
However, in vitro androgenesis can be highly stressful, which could result in different genetic and epigenetic changes in derived plants (Oleszczuk et al.2014; Machczyńska et al.2015). For example, aneuploidy among androgenic progeny of triticale was reported (Oleszczuk et al.2011). Chromosome aberrations, including translocations, in androgenic triticale were observed, as well (Oleszczuk et al.2011). Such aberrations are heritable and provide phenotypic changes, which can decrease the value of triticale cultivars (Małuszyńska et al.2001). It is reported from breeders that triticale DH lines produce off-type individuals, and the phenotypic alternations are mainly connected with spike morphology (Banaszak et al.2007).
The morphology of the spike (inflorescence) of small-grain cereals, including triticale, is a key factor in determining grain yield. The components of the spike (e.g. spikelets, glumes, and lemma) influence each other. The number and arrangement of spike components affect spike length, grain number per spike, and spikelet number per spike, which all contribute to final grain yield per spike (Guo et al.2016). To produce varieties with the most efficient grain production in different environments, the uniformity of spike morphology is the most important issue.
The objectives of this research are to determine whether the changes in the spike morphology of androgenic progeny of triticale DH 371/14 line (developed by DANKO Plant Breeding Ltd. in Choryń, Poland) are the consequences of chromosome alternations? For this purpose, (1) we analyzed the chromosome sets for 121 randomly chosen offspring plants (C1) derived from double haploid plants (C0), and (2) we assessed the alternations of spike morphology for C1 plants.
Materials and Methods
Plant material
The offspring plants (C1) of DH 371/14 triticale line were analyzed in this study. The triticale double haploid line used in the experiments (C0) was developed from DANKO 371/14 strain (F6 generation) through anther cultures. This complex hybrid was derived by (LAD 54/04 × Silverado) × Twingo cross-hybridizations, made in 2009. LAD 54/04 strain was developed through (Fidelio × LAD 340/94) × Magnat hybridizations.
Chromosome preparation
One hundred and twenty-one seeds were germinated on Petri dishes, in the dark, at room temperature. After 3 d, the root tips were collected. Next, the seedlings were transferred into the vernalization chamber (8 wk; 4°C). After that, the plants were grown in the greenhouse at Poznan University of Life Sciences, Dept. of Genetics and Plant Breeding (day/night photoperiod 16 h/8 h; 16–20°C) until harvest. Accumulation and fixation of mitotic chromosomes were carried out according to Kwiatek et al. (2017). The digestion was performed in 0.2% (v/v) Onozuka R-10 and Calbiochem cellulase (1:1 ratio) and 20% pectinase (Merck KGaA, Darmstadt, Germany) in 10 mM citrate buffer (pH 4.6) at 37°C for 2 h and 40 min. Mitotic chromosome spreads were prepared as described by Heckmann et al. (2014) and Kwiatek et al. (2017) with minor modifications.
Molecular probe preparation and fluorescence in situ hybridization
The genomic DNA of rye (R-genome; cv. “Dankowskie Złote”) and durum wheat (A- and B-genomes; cv. “Ceres”) was isolated using Plant & Fungi DNA Purification Kit (Eurx, Poland). Total genomic DNA of rye was labeled by nick translation with Atto-550 dye (Atto-550NT kit; Jena Bioscience). Blocking DNA from durum wheat was sheared by boiling for 30–45 min and used at a ratio of 1:50 (probe DNA to blocking DNA). Genomic in situ hybridization (GISH) or multicolor GISH was performed according to previously published protocol (Kwiatek et al.2016a). Mitotic chromosomes were identified using fluorescence in situ hybridization (FISH) with the repetitive sequences from pTa-86, pTa-535, and pTa-k374 (25S rDNA) clones as well as pTa-103 clone (containing centromere specific sequence), characterized by Komuro et al. (2013), amplified from genomic DNA of wheat (Chinese Spring) according to Kwiatek et al. (Kwiatek et al.2016b), and labeled by nick translation kits (Jena Bioscience) using Atto-488, Atto-550, and Atto-647 dyes. FISH was performed according to Kwiatek et al. (2016b). Slides were analyzed with the help of Axio Observer version 7 (Carl Zeiss, Oberkochen, Germany) inverted fluorescence microscope. Image processing was done using ZEN Pro software (Carl Zeiss) imaging software and PaintShop Pro X5 software (version 15.0.0.183; Corel Corporation, Ottawa, Canada). Identification of chromosomes was carried out by comparing the signal patterns of the probes (Komuro et al.2013; Kwiatek et al.2016b, 2017).
Evaluation spike morphology traits
Spike morphology traits were analyzed in all 118 of 121 plants (3 plants were rejected on the basis of cytological evaluation) which were the progeny of DH 371/14 and have been evaluated in FISH/GISH experiments. The following quantitative traits have been evaluated: spike length (SL), number of spikes per plant (SPP), number of spikelets per spike (SplPS), and number of grains per spike (GPS). Analyses of variance (ANOVA) for each trait data according to random model compared between different karyotypic variants by the use of Tukey’s honest significant difference (HSD) test at P = 0.05 and P = 0.01 significance levels (STATISTICA 13.1, StatSoft Poland).
Results
Chromosome identification
C0 plants of DH 371/14 line plants were harvested, and 121 randomly selected seeds of C1 generation (obtained by self-pollination of the C0 plants) were used for further investigations, as follows: (1) karyotype evaluation and (2) spike morphology analyses. At first, two rounds of in situ hybridization (ISH) were performed. Genomic in situ hybridization (GISH) was used to categorize the chromosomes according to their genomic belonging (genomes: A, B, and R). For identification of centric breaks, a centromere specific probe pTa-103 was used (Fig. 1a). Following FISH, experiments were conducted after GISH scoring and reprobing. A combination of three probes (pTa-86, pTa-535, and pTa-k374) was sufficient to identify all chromosomes of triticale. The structural abnormalities or disorders of chromosome number were observed only among R-genome chromosomes. GISH/FISH experiments allow distinguishing four groups of karyotypes (Fig. 1). The first group consisted of 9 plants and each plant carried 44 chromosomes. Within this group, 2R chromosomes were represented by double ditelosomics 2RS and 2RL (Fig. 1a). The second group contained 12 plants and each of them carried 40 chromosomes. The lack of 2R chromosome pair was their common feature (2R nullisomy—N2R) (Fig. 1b, c). The third group included 19 nullisomic plants with the lack of 5R chromosomes (N5R; 40 chromosomes) (Fig. 1d, e). The fourth group counted 78 plants with 42 chromosomes (Fig. 1f, g). Plants within this group did not perform any chromosomal aberrations or disorders of chromosome number (“normal” karyotype). Additionally, the FISH/GISH examination enabled to identify one plant with 2BS.4RL chromosome translocation, one plant had an additional B-genome chromosome fragment, and another one possessed telosomic 5BL chromosomes. However, these three latter plants were not included within any group and excluded from the following spike morphology evaluation.
Chromosome sets of followed triticale plants: (a) double ditelosomic d2RS + d2RL analyzed by fluorescence/genomic in situ hybridization, using genomic probes (A- and B-genome—blue; R-genome—red) and pTa-103 centromere-specific probe (green); (b, c) nullisomic N2R; (d, e) nullisomic N5R; (f, g) “normal”—complete chromosome set and analyzed by genomic in situ hybridization using genomic probes (A-genome—green; B-genome—blue; and R-genome—red) followed by fluorescence in situ hybridization, using pTa-86 (green), pTa-535 (red), and pTa-k374 (25S rDNA; yellow) probes.
Evaluation spike morphology traits
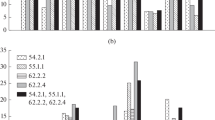
Quantitative spike traits have been evaluated for 118 of 121 plants of DH 371/14 progeny, which were included into 4 groups of karyotypes. The remaining three plants were rejected. We have a closer look on the following: number of SPP (Fig. 2a), SL (Fig. 2b), number of SplPS (Fig. 2c), and number of GPS (Fig. 2d). The differences between the four groups of karyotypes connected with the number of spikes per plant were not significant (Table 1). The means of SPP ranged from 2.89 to 3.86. The variance analyses of the spike length scores showed several significant differences. The group means of spike length ranged from 4.38 to 13.98 cm. Tukey’s honest significant difference test was P0.01 = 1.24 and revealed significant differences in spike length comparing plants with and without chromosome aberrations. Similar dependencies between groups were observed considering number of spikelets per spike and number of grains per spike. Again, analysis of variance followed by Tukey’s honest significant difference test showed the significant differences between plants with “normal” karyotype versus nullisomic (N2R or N5R) plants, when SpPS and GPS characteristics were considered. Looking into SL, SpPS, and GPS characteristics, there were no significant differences between plants with complete chromosome set compared to double ditelosomics (d2RS + d2RL).
Discussion
Fluorescence in situ hybridization (FISH) and genomic in situ hybridization (GISH) have been applied to board spectrum of analyses, including studies of interspecific hybridization and polyploidy and analyses of phylogenetic relationships between species, genetic mapping, and analysis of plant breeding materials (Chester et al.2010). ISH techniques are the most suitable and rapid methods for the identification of chromosomal disorders (Kwiatek and Nawracała 2018; Kwiatek et al.2019). Chromosome-specific FISH markers are considered as powerful tools for evaluation of genomic changes. Moreover, there are several highly conserved sequences that can be used as probes across most plants, for example, 5S rDNA, 35S rDNA, and telomere repeats.
In this study, we have used a combination of FISH and GISH methods to investigate the chromosomal aberrations in androgenic offspring of triticale breeding line DH 371/14 provided by the breeding company (DANKO Hodowla Roślin Sp. z o.o.). It was interesting that among 31 nullisomic C1 plants (~ 25%), only 2R or 5R chromosomes were involved in nullisomy. Other nine plants (~ 7%) carried 2R chromosomes, which were broken in centromeres. Chromosome aberrations involving 5R chromosomes among androgenic progeny of hexaploid triticale were previously reported by Charmet et al. (1986) and Oleszczuk et al. (2014). The high frequency of aneuploids could be genotype-specific. Oleszczuk et al. (2011) reported that aneuploidy appears to be the most frequent in the most recalcitrant combinations. Such disorders could appear because it eliminates chromosomes that possess loci of genes, which prevent the switch from the gametophytic to the sporophytic microspore (Oleszczuk et al.2011). This could be connected with the fact that 2R and 5R chromosomes are not involved in the stimulation of androgenesis. González et al. (2005) founded that 50.7% of QTLs connected with androgenic response are located in R-genome of triticale. The major QTLs, significantly associated with green plant regeneration, is located on chromosome 3R (González et al.2005). Other QTLs, connected with final androgenic response (number of regenerated plantlets from 1000 anthers), are located on chromosomes 1B, 1R, 4R, and 7R (González et al.2005). Moreover, Lazar et al. (1987) observed the effects of rye chromosomes on androgenesis in cultured anthers of wheat–rye addition lines and reported that chromosomes 1R and 4R enhance the production of haploid embryos. However, none of QTLs responsible for androgenic response is identified on 2R, 5R, and 6R chromosomes. Hence, it could be hypothesized that aberrations of 2R, 5R, and 6R chromosomes do not disrupt the regeneration of androgenic plants. Additionally, Dobrovolskaya et al. (2003) studied the effects of rye chromosomes 1R and 5R on androgenesis in cultured anthers of wheat–rye substitution lines and founded that chromosome 1R stimulated embryogenesis and chromosome 5R suppressed this process.
The remaining questions are as follows: (1) what is the reason for chromosome aberrations in androgenic triticale and (2) why those mutations are mostly connected with R-genome chromosomes of triticale? There are several hypotheses on the reasons for instability of chromosome structure and constitution in triticale, which were reviewed by Kaltsikes (1974). One of them is the theory of genomic disharmony, which means the inability of the two parental genomes (wheat and rye) to coordinate their activities or works in unison within the common nucleus. Another theory, called allocycly, states that the two genomes are replicating their DNA at different times. Precocious separation of bivalents is considered as another theory for chromosome structure and constitution instability in triticale. The reduction in pairing observed was attributed by Li et al. (2015) regarding termination and failure of chiasmata after diakinesis. Another possible reason of chromosome instability in triticale could be connected with meiotic irregularities, which might be related to the ratio of rye to wheat genomes in the amphiploid (Muntzing et al.1963). This hypothesis is based on the observations on triticale of the hexaploid, octoploid, and decaploid levels, which indicated that as the ratio of wheat to rye genomes moved from 2:1 to 3:1 and 3:2, irregularities increased. Recent cytogenetic studies of wheat–rye introgression forms showed that the bivalent formation is crucial for success of proper meiotic division. Bivalent formation requires that homologous chromosomes, mainly located in separate nuclear territories in premeiotic interphase nuclei (Bass et al.2000), move to find one another. In many species, concomitant with a chromatin remodeling process that causes a considerable chromosome elongation during leptotene, telomeres undergo an oriented migration and converge in a tight cluster in a small area of the nuclear envelope. This meiotic configuration, the so-called bouquet, facilitates chromosome interactions that culminate in the identification of the homologous partner (Bass et al.2000). The bouquet is disorganized once homologs undergo synapsis. So, the identification of the homologous partner in the invaded chromatid is necessary for chromosome pairing and synapsis in many organisms. Then, homologs become aligned, form the tripartite synaptonemal complex (SC) during zygotene, and are fully synapsed at pachytene (Page and Hawley 2004). The SC maintains homologs in close juxtaposition along their length and serves as a scaffold for factors of the recombination repairing machinery.
Naranjo (2018) examined the meiotic process in seven wheat–rye introgression lines, where each carrying one of the seven chromosome pairs of rye (S. cereale, 2n = 14) transferred into hexaploid wheat T. aestivum. He reported that chromosome 4R increased its length in early prophase I much more than other chromosomes of rye. Moreover, he showed that telomere of 4RS arm failed convergence, but developed normal synapsis. In his opinion, it is a result of the strong increase of its length in early prophase I that facilitated homologous encounters in intercalary regions. What is interesting, chiasma frequencies in both arms of 4R were reduced. Similarly, the short arm of metacentric chromosome 2R often failed to form chiasmata despite normal synapsis. Other rye chromosomes (1R, 3R, and 7R) showed a regular meiotic behavior (Naranjo 2018).
This work was further elaborated by Perničková et al. (2019a, b). Their results showed that alien chromosomes show reduced meiotic pairing relative to the host genome and may be eliminated over generations. Perničková and collaborators (Perničková et al. 2019a, b) observed reduced pairing, which appeared to result from a failure of some telomeres of alien chromosomes to incorporate into the leptotene bouquet at the onset of meiosis, thereby preventing chiasmate pairing. They analyzed somatic nuclei of wheat with rye chromosome introgressions using 3D-FISH and found that the telomeres of rye chromosomes or chromosome arms frequently occupied positions away from the nuclear periphery. The frequencies of such abnormal telomere positioning were similar to the frequencies of out-of-bouquet telomere positioning at leptotene and of pairing failure at metaphase I. Perničková et al. (2019a, b) suggested that the improper positioning of alien chromosomes that leads to reduced pairing is not a strictly meiotic event but rather a consequence of a more systemic problem. They postulated that improper positioning in the nuclei probably impacts the ability of alien chromosomes to migrate into the telomere bouquet at the onset of meiosis, preventing synapsis and chiasma establishment and leading to their gradual elimination over generations. Moreover, they confirmed the effect of telomere clustering during meiosis and even speculated that the proper positioning in somatic cells is prerequisite for the initiation of synapsis and correct pairing and division.
There are other alternative methods, which are widely applied to uncover the chromosome disorders. The highly specific rearrangements that result from chromosomal translocations can be identified with reverse transcription polymerase chain reaction (RT-PCR) analysis. The PCR technique uses specific synthetic primers to amplify a section of a gene in vitro. PCR can be carried out on RNA following reverse transcription (mRNA → cDNA) (Bridge 2008). However, compared with cytogenetic approaches, one of the disadvantages of RT-PCR analysis is the inability to detect chromosome breaks. Modern methods of molecular biology, including next-generation sequencing (NGS) and bioinformatics, now provide an alternative way to rapidly identify various types of sequences suitable for FISH at modest cost. NGS provides large amounts of data. In higher plant, non-coding simple sequence repeats are the most numerous fractions of DNA sequences in a broad range of species and have always presented technical challenges for sequence alignment and assembly programs. There are several strategies used by current bioinformatics systems to solve obstacles in alignment and assembly, which, in turn, can produce biases and errors when interpreting results (Treangen and Salzberg 2011). When NGS approaches are combined with FISH, it is possible to rapidly characterize genomes. Swaminathan et al. (2007) first used this approach for soybean and identified high-copy repeat families, such as telomeric and ribosomal DNA and also repeats with potential use as FISH probes, including TEs, centromeric, and telomere-associated sequences. With molecular cytogenetic analysis, all major chromosomal abnormalities may be uncovered. FISH can be informative in both metaphase and interphase cell preparations. It can assist in identification of marker chromosomes, ring chromosomes, and chromosomal rearrangements that are diagnostically useful both in breeding and considering fundamental research.
In our study, the nullisomy of chromosome 2R and 5R resulted in vast changes in spike morphology of triticale plants. The spikes of nullisomic plants had significantly decreased spike length which correlated with the reduction of number of spikelets per spike and number of grains per spike. It could be explained that several of major QTLs connected with spike morphology is located on chromosomes 2R and 5R. Börner et al. (2000) mapped major QTLs connected with the following: number of grains per spike (on 2R and 5R), thousand grains mass (2R), ear yield (2R and 5R), number of spikelets per plant (2R), and peduncle length (5R). Myśków et al. (2014) located QTLs for number of spikelets per spike on chromosome 2R of rye. Moreover, the Mrs1 gene, which governs spike branching, is located on 2R chromosome, as well (Dobrovolskaya et al.2009).
One of the most challenging efforts for breeding is the uniformity of triticale cultivars. It is said that chromosome aberration, including nullisomy, is a bothering problem in triticale breeding. This crop, like no other, suffers chromosome pairing issues and tends to show a fair proportion of aneuploids among sexually derived and androgenic offspring (Oleszczuk et al.2011). Meiotic instability usually results in structural and numerical aberrations of triticale chromosomes, which are correlated with yield reduction, which was showed in this work. Hence, meiotic abnormalities are obstacles to commercial exploitation of triticale. Taking this into consideration, there is a need to conduct further studies, which will aim in detailed exploration of meiosis in triticale and double haploids derived from this crop by targeting meiosis progress. The main question is what are the reasons of changes in chromosome structure and constitution in triticale? This issue is also interesting, considering allopolyploidization, which is a key mechanism of evolution of higher plants.
References
Banaszak Z, Kaźmierczak P, Trąbka A, Apolinarska B, Bocianowski J (2007) Wpływ wybranych czynników na wyrównanie odmian pszenżyta [The influence of some factors on uniformity of triticale cultivars]. Biul Inst Hod i Aklim Roślin 244:145–154
Bass HW, Riera-Lizarazu O, Ananiev EV, Bordoli SJ, Rines HW, Phillips RL, Sedat JW, Agard DA, Cande WZ (2000) Evidence for the coincident initiation of homolog pairing and synapsis during the telomere-clustering (bouquet) stage of meiotic prophase. J Cell Sci 113:1033–1042
Börner A, Korzun V, Voylokov AV, Worland AJ, Weber WE (2000) Genetic mapping of quantitative trait loci in rye (Secale cereale L.). Euphytica 116:203–209. https://doi.org/10.1023/A:1004052505692
Bridge JA (2008) Advantages and limitations of cytogenetic, molecular cytogenetic, and molecular diagnostic testing in mesenchymal neoplasms. J Orthop Sci 13:273–282. https://doi.org/10.1007/s00776-007-1215-1
Charmet G, Bernard S, Bernard M (1986) Origin of aneuploid plants obtained by anther culture in triticale. Can J Genet Cytol 28:444–452. https://doi.org/10.1139/g86-067
Chester M, Leitch AR, Soltis PS, Soltis DE (2010) Review of the application of modern cytogenetic methods (FISH/GISH) to the study of reticulation (polyploidy/hybridisation). Genes (Basel) 1:166–192. https://doi.org/10.3390/genes1020166
Dobrovolskaya O, Martinek P, V Voylokov A, Korzun V, Röder M, Börner A (2009) Microsatellite mapping of genes that determine supernumerary spikelets in wheat (T. aestivum) and rye (S. cereale). Theor Appl Genet 119:867–874. https://doi.org/10.1007/s00122-009-1095-1
Dobrovolskaya OB, Pershina LA, Kravtsova LA, Shchapova AI (2003) Comparative effects of rye chromosomes 1R and 5R on androgenesis in cultured anthers of wheat-rye substitution lines as dependent on the line origin. Russ J Genet 39:467–470. https://doi.org/10.1023/A:1023378219158
Dwivedi SL, Britt AB, Tripathi L, Sharma S, Upadhyaya HD, Ortiz R (2015) Haploids: constraints and opportunities in plant breeding. Biotechnol Adv 33:812–829. https://doi.org/10.1016/j.biotechadv.2015.07.001
Eudes F, Chugh A (2009) An overview of triticale doubled haploids BT - advances in haploid production in higher plants. In: Touraev A, Forster BP, Jain SM (eds) . Springer Netherlands, Dordrecht, pp 87–96
FAO (2016) FAO statistics. In: Food Agric. Organ. United Nations
González JM, Muñiz LM, Jouve N (2005) Mapping of QTLs for androgenetic response based on a molecular genetic map of × Triticosecale Wittmack. Genome 48:999–1009. https://doi.org/10.1139/g05-064
Guo Z, Chen D, Alqudah AM, Röder MS, Ganal MW, Schnurbusch T (2016) Genome-wide association analyses of 54 traits identified multiple loci for the determination of floret fertility in wheat. New Phytol 214:257–270. https://doi.org/10.1111/nph.14342
Heckmann S, Jankowska M, Schubert V, Kumke K, Ma W, Houben A (2014) Alternative meiotic chromatid segregation in the holocentric plant Luzula elegans. Nat Commun 5:4979. https://doi.org/10.1038/ncomms5979
Kaltsikes PJ (1974) Univalency in triticale. In: Maclntyre R, Campbell M (eds) Triticale. Proceedings of an international symposium El Batan, Mexico, 1–3 October 1973. International Development Research Centre, Ottawa, pp 159–167
Komuro S, Endo R, Shikata K, Kato A (2013) Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 56:131–137. https://doi.org/10.1139/gen-2013-0003
Kwiatek M, Belter J, Majka M, Wiśniewska H (2016a) Allocation of the S-genome chromosomes of Aegilops variabilis Eig. carrying powdery mildew resistance in triticale (× Triticosecale Wittmack). Protoplasma 253:329–343. https://doi.org/10.1007/s00709-015-0813-6
Kwiatek M, Majka M, Majka J, Belter J, Suchowilska E, Wachowska U, Wiwart M, Wiśniewska H (2016b) Intraspecific polymorphisms of cytogenetic markers mapped on chromosomes of Triticum polonicum L. PLoS One 11:e0158883. https://doi.org/10.1371/journal.pone.0158883
Kwiatek MT, Kurasiak-Popowska D, Mikołajczyk S, Niemann J, Tomkowiak A, Weigt D, Nawracała J (2019) Cytological markers used for identification and transfer of Aegilops spp. chromatin carrying valuable genes into cultivated forms of Triticum. Comp Cytogenet 13:2–19
Kwiatek MT, Nawracała J (2018) Chromosome manipulations for progress of triticale (×Triticosecale) breeding. Plant Breed 137:823–831. https://doi.org/10.1111/pbr.12652
Kwiatek MT, Wiśniewska H, Ślusarkiewicz-Jarzina A, Majka J, Majka M, Belter J, Pudelska H (2017) Gametocidal factor transferred from Aegilops geniculata Roth can be adapted for large-scale chromosome manipulations in cereals. Front Plant Sci 8:409. https://doi.org/10.3389/fpls.2017.00409
Lazar MD, Chen THH, Scoles GJ, Kartha KK (1987) Immature embryo and anther culture of chromosome addition lines of rye in Chinese spring wheat. Plant Sci 51:77–81. https://doi.org/10.1016/0168-9452(87)90222-6
Li H, Guo X, Wang C, Ji W (2015) Spontaneous and divergent hexaploid triticales derived from common wheat × rye by complete elimination of D-genome chromosomes. PLoS One 10:e0120421. https://doi.org/10.1371/journal.pone.0120421
Machczyńska J, Zimny J, Bednarek PT (2015) Tissue culture-induced genetic and epigenetic variation in triticale (× Triticosecale spp. Wittmack ex A. Camus 1927) regenerants. Plant Mol Biol 89:279–292. https://doi.org/10.1007/s11103-015-0368-0
Małuszyńska E, Drzewiecki J, Łapiński B (2001) Tożsamość nietypowych roślin pszenżyta na podstawie analizy elektroforetycznej i cytogenetycznej ziarniaków [Identity of triticale off-type plants on the basis of electrophoretic and cytogenetic analysis of kernels]. Biul IHAR 218/219:251–259
Muntzing A, Hrishi NJ, Tarkowski C (1963) Rversion to haploidy in strains of hexaploid and octoploid triticale. Hereditas 49:78–90. https://doi.org/10.1111/j.1601-5223.1963.tb01869.x
Myśków B, Hanek M, Banek-Tabor A, Maciorowski R, Stojałowski S (2014) The application of high-density genetic maps of rye for the detection of QTLs controlling morphological traits. J Appl Genet 55:15–26. https://doi.org/10.1007/s13353-013-0186-5
Naranjo T (2018) Variable patterning of chromatin remodeling, telomere positioning, synapsis, and chiasma formation of individual rye chromosomes in meiosis of wheat-rye additions. Front Plant Sci 9:880. https://doi.org/10.3389/fpls.2018.00880
Oettler G, Tams SH, Utz HF, Bauer E, Melchinger AE (2005) Prospects for hybrid breeding in winter triticale. Crop Sci 45:1476–1482. https://doi.org/10.2135/cropsci2004.0462
Oleszczuk S, Rabiza-Swider J, Zimny J, Lukaszewski AJ (2011) Aneuploidy among androgenic progeny of hexaploid triticale (X Triticosecale Wittmack). Plant Cell Rep 30:575–586. https://doi.org/10.1007/s00299-010-0971-0
Oleszczuk S, Tyrka M, Zimny J (2014) The origin of clones among androgenic regenerants of hexaploid triticale. Euphytica 198:325–336. https://doi.org/10.1007/s10681-014-1109-1
Page SL, Hawley RS (2004) The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20:525–558. https://doi.org/10.1146/annurev.cellbio.19.111301.155141
Pauk J, Puolimatka M, Lökös Tóth K, Monostori T (2000) In vitro androgenesis of triticale in isolated microspore culture. Plant Cell Tissue Organ Cult 61:221–229. https://doi.org/10.1023/A:1006416116366
Perničková K, Koláčková V, Lukaszewski AJ, Fan C, Vrána J, Duchoslav M, Jenkins G, Phillips D, Šamajová O, Sedlářová M, Šamaj J, Doležel J, Kopecký D (2019a) Instability of alien chromosome introgressions in wheat associated with improper positioning in the nucleus. Int J Mol Sci 20:1448. https://doi.org/10.3390/ijms20061448
Perničková K, Linc G, Gaal E, Kopecky D, Samajova O, Lukaszewski AJ (2019b) Out-of-position telomeres in meiotic leptotene appear responsible for chiasmate pairing in an inversion heterozygote in wheat (Triticum aestivum L.). Chromosoma 128:31–39. https://doi.org/10.1007/s00412-018-0686-5
Ślusarkiewicz-Jarzina A, Pudelska H, Woźna J, Pniewski T (2017) Improved production of doubled haploids of winter and spring triticale hybrids via combination of colchicine treatments on anthers and regenerated plants. J Appl Genet 58:287–295. https://doi.org/10.1007/s13353-016-0387-9
Swaminathan K, Varala K, Hudson ME (2007) Global repeat discovery and estimation of genomic copy number in a large, complex genome using a high-throughput 454 sequence survey. BMC Genomics 8:132. https://doi.org/10.1186/1471-2164-8-132
Thomas WTB, Forster BP, Gertsson B (2003) Doubled haploids in breeding BT - doubled haploid production in crop plants: a manual. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) . Springer Netherlands, Dordrecht, pp 337–349
Treangen TJ, Salzberg SL (2011) Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet 13:36–46. https://doi.org/10.1038/nrg3117
Tuvesson S, von Post R, Ljungberg A (2003) Triticale anther culture BT - doubled haploid production in crop plants: a manual. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) . Springer Netherlands, Dordrecht, pp 117–121
Weigt D, Kiel A, Nawracała J, Pluta M, Łacka A (2016) Solid-stemmed spring wheat cultivars give better androgenic response than hollow-stemmed cultivars in anther culture. In Vitro Cell Dev Biol Plant 52:619–625. https://doi.org/10.1007/s11627-016-9793-2
Weigt D, Kiel A, Nawracała J, Tomkowiak A, Kurasiak-Popowska D, Siatkowski I, Ługowska B (2017) Obtaining doubled haploid lines of the Lr19 gene using anther cultures of winter wheat genotypes. BioTechnologia 97:285–293. https://doi.org/10.5114/bta.2016.64545
Wilson A (1876) Wheat and rye hybrids. Edinb Bat Sac Trans 12:286–288
Wiśniewska H, Majka M, Kwiatek M, Gawłowska M, Surma M, Adamski T, Kaczmarek Z, Drzazga T, Ługowska B, Korbas M, Belter J (2019) Production of wheat-doubled haploids resistant to eyespot supported by marker-assisted selection. Electron J Biotechnol 37:11–17. https://doi.org/10.1016/j.ejbt.2018.10.003
Acknowledgments
We thank Ms. Daria Dec, Ms. Zofia Drozdowska, Ms. Aleksandra Noweiska, and Ms. Paulina Lipińska for invaluable assistance in evaluation of spike morphology traits. In addition, we would like to thank all of the reviewers and manuscript editor for their careful review of the manuscript and for their excellent suggestions for improving our initial work.
Funding
This publication is being co-financed by the framework of Ministry of Science and Higher Education program as “Regional Initiative Excellence” in years 2019-2022, project no. 005/RID/2018/19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Yong Eui Choi
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kwiatek, M.T., Banaszak, Z., Skowrońska, R. et al. Spike morphology alternations in androgenic progeny of hexaploid triticale (× Triticosecale Wittmack) caused by nullisomy of 2R and 5R chromosomes. In Vitro Cell.Dev.Biol.-Plant 56, 150–158 (2020). https://doi.org/10.1007/s11627-019-10021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-10021-7