Abstract
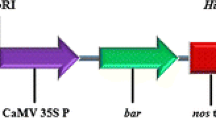
An improved protocol for Agrobacterium-mediated transformation of an elite, mature black cherry genotype was developed. To increase transformation efficiency, vacuum infiltration, sonication, and a combination of the two treatments were applied during the cocultivation of leaf explants with Agrobacterium tumefaciens strain EHA105 harboring a PsAGAMOUS RNAi plasmid (pART27-PsAGRNAi). The effects of Agrobacterium culture density and cocultivation duration on transformation efficiency were examined using EHA105 harboring either pBI121-MDL4 or pBI121-PsTFL1. In addition, the effect of the binary vector on transformation efficiency was also studied. Fifteen-minute vacuum infiltration without sonication produced the highest transformation efficiency (21.7%) in experiments using pART27-PsAGRNAi. OD600 values of 1.0 and 1.5 resulted in a transformation efficiency of 5% when pBI121-PsTFL1 was used for transformation. Transformation efficiency of 5% was also obtained from 3-d cocultivation using construct pBI121-MDL4 whereas no shoots regenerated after 4-d cocultivation. The binary vectors used also impacted transformation efficiency. PCR and quantitative-PCR analyses were used to confirm the integration of transgenes and determine the copy number of the selectable marker gene, neomycin phosphotransferase II, in 18 putative transgenic lines. Rooting of transgenic black cherry shoots was achieved at a frequency of 30% using half-strength Murashige and Skoog medium supplemented with 2% sucrose, 5 μM naphthaleneacetic acid, 0.01 μM kinetin, and 0.793 mM phloroglucinol, and the resulting transgenic plants were successfully acclimatized.




Similar content being viewed by others
References
Abou-Alaiwi WA, Potlakayala SD, Goldman SL, Josekutty PC, Karelia DN, Rudrabhatla SV (2012) Agrobacterium-mediated transformation of the medicinal plant Centaurea montana. Plant Cell Tissue Organ Cult 109:1–8
Amoah BK, Wu H, Sparks C, Jones HD (2001) Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot 52:1135–1142
Bakshi S, Sadhukhan A, Mishra S, Sahoo L (2011) Improved Agrobacterium-mediated transformation of cowpea via sonication and vacuum infiltration. Plant Cell Rep 30:2281–2292
Barnd B, Ginzel MD (2008) Causes of gummosis in black cherry (Prunus serotina). USDA Forest Service and Purdue University, FNR–229–W. Purdue University, West Lafayette, IN
Caboni E, Tonelli MG, Lauri P, Iacovacci P, Kevers C, Damiano C, Gaspar T (1997) Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol Plant 39:91–97
Cassens DL (2007) Hardwood lumber and veneer series: black cherry. Purdue University Extension, FNR–276–W. Purdue University, West Lafayette, IN
Charity JA, Holland L, Donaldson SS, Grace L, Walter C (2002) Agrobacterium-mediated transformation of Pinus radiata organogenic tissue using vacuum-infiltration. Plant Cell Tissue Organ Cult 70:51–60
Chen PY, Wang CK, Soong SC, To KY (2003) Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol Breed 11:287–293
De Clercq J, Zambre M, Van Montagu M, Dillen W, Angenon G (2002) An optimized Agrobacterium-mediated transformation procedure for Phaseolus acutifolius A. Gray. Plant Cell Rep 21:333–340
de Oliveira MLP, Febres VJ, Costa MGC, Moore GA, Otoni WC (2009) High-efficiency Agrobacterium-mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Rep 28:387–395
Druart P, Kevers C, Boxus P, Gaspar T (1982) In vitro promotion of root formation by apple shoots through darkness effect on endogenous phenols and peroxidases. Z Pflanzenphysiol 108:429–436
Du N, Pijut PM (2008) Regeneration of plants from Fraxinus pennsylvanica hypocotyls and cotyledons. Sci Hortic 118:74–79
Dutt M, Grosser JW (2009) Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue Organ Cult 98:331–340
Espinosa AC, Pijut PM, Michler CH (2006) Adventitious shoot regeneration and rooting of Prunus serotina in vitro cultures. Hort Sci 41:193–201
Fett-Neto AG, Fett JP, Goulart LWV, Pasquali G, Termignoni RR, Ferreira AG (2001) Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol 21:457–464
Gleave AP (1992) A versatile binary vector system with a T-DNA organizational structure conductive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207
Hammatt N, Grant NJ (1997) Micropropagation of mature British wild cherry. Plant Cell Tissue Organ Cult 47:103–110
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Hu ZH, Poulton JE (1999) Molecular analysis of (R)-(+)-mandelonitrile lyase microheterogeneity in black cherry. Plant Physiol 119:1535–1546
Jin S, Zhang X, Liang S, Nie Y, Guo X, Huang C (2005) Factors affecting transformation efficiency of embryogenic callus of upland cotton (Gossypium hirsutum) with Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 81:229–237
Li ZT, Jayasankar S, Gray DJ (2004) Bi-directional duplex promoters with duplicated enhancers significantly increase transgene expression in grape and tobacco. Transgenic Res 13:143–154
Liu X, Anderson JM, Pijut PM (2010) Cloning and characterization of Prunus serotina AGAMOUS, a putative flower homeotic gene. Plant Mol Biol Rep 28:193–203
Liu X, Pijut PM (2008) Plant regeneration from in vitro leaves of mature black cherry (Prunus serotina). Plant Cell Tissue Organ Cult 94:113–123
Liu X, Pijut PM (2010) Agrobacterium-mediated transformation of mature Prunus serotina (black cherry) and regeneration of transgenic shoots. Plant Cell Tissue Organ Cult 101:49–57
Liu Z, Park B-J, Kanno A, Kameya T (2005) The novel use of a combination of sonication and vacuum infiltration in Agrobacterium-mediated transformation of kidney bean (Phaseolus vulgaris L.) with lea gene. Mol Breed 16:189–197
Marion J, Bach L, Bellec Y, Meyer C, Gissot L, Faure JD (2008) Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. Plant J 56:169–179
Marquis DA (1990) Prunus serotina Ehrh. Black Cherry. In: Burns RM and Honkala BH (tech. coords.) Silvics of North America: Vol. 2, Hardwoods. Agricultural Handbook 654 (2nd ed.). U.S. Dept. of Agriculture, Forest Service, Washington, DC, pp. 594–604
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray MG, Thompson WF (1980) Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Padilla IMG, Webb K, Scorza R (2003) Early antibiotic selection and efficient rooting and acclimatization improve the production of transgenic plum plants (Prunus domestica L.). Plant Cell Rep 22:38–45
Park B-J, Liu Z, Kanno A, Kameya T (2005) Transformation of radish (Raphanus sativus L.) via sonication and vacuum infiltration of germinated seeds with Agrobacterium harboring a group 3 LEA gene from B. napus. Plant Cell Rep 24:494–500
Peña L, Séguin A (2001) Recent advances in the genetic transformation of trees. Trends Biotechnol 19:500–506
Pérez-Clemente RM, Perez-Sanjuan A, Garcia-Ferriz L, Beltran JP, Canas LA (2004) Transgenic peach plants (Prunus persica L.) produced by genetic transformation of embryo sections using the green fluorescent protein (GFP) as an in vivo marker. Mol Breed 14:419–427
Petri C, Scorza R (2010) Factors affecting adventitious regeneration from in vitro leaf explants of 'Improved French' plum, the most important dried plum cultivar in the USA. Ann Appl Biol 156:79–89
Petri C, Webb K, Hily JM, Dardick C, Scorza R (2008) High transformation efficiency in plum (Prunus domestica L.): a new tool for functional genomics studies in Prunus spp. Mol Breed 22:581–591
SAS Institute (1999) Version 9.2. SAS Institute, Cary, NC
Subramanyam K, Subramanyam K, Sailaja KV, Srinivasulu M, Lakshmidevi K (2011) Highly efficient Agrobacterium-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. Plant Cell Rep 30:425–436
Teixeira da Silva JA, Dobránszki J, Ross S (2013) Phloroglucinol in plant tissue culture. In Vitro Cell Dev Biol Plant 49:1–16
Trick HN, Finer JJ (1997) SAAT: sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–336
Wang Y, Pijut PM (2013) Isolation and characterization of a TERMINAL FLOWER 1 homolog from Prunus serotina Ehrh. Tree Physiol 33:855–865
Weng H, Pan A, Yang L, Zhang C, Liu Z, Zhang D (2004) Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay with HMG I/Y as an endogenous reference gene. Plant Mol Biol Rep 22:289–300
Wu H, Sparks C, Amoah B, Jones HD (2003) Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep 21:659–668
Xing Y, Yang Q, Ji Q, Luo Y, Zhang Y, Gu K, Wang D (2007) Optimization of Agrobacterium-mediated transformation parameters for sweet potato embryogenic callus using β-glucuronidase (GUS) as a reporter. Afr J Biotechnol 6:2578–2584
Yang Y, Bao M, Liu G (2010) Factors affecting Agrobacterium-mediated genetic transformation of embryogenic callus of Parthenocissus tricuspidata Planch. Plant Cell Tissue Organ Cult 102:373–380
Zhang L, Chin DP, Mu M (2010) Agrobacterium-mediated transformation of protocorm-like bodies in Cattleya. Plant Cell Tissue Organ Cult 103:41–47
Zimmerman RH (1984) Rooting apple cultivars in vitro: Interactions among light, temperature, phloroglucinol and auxin. Plant Cell Tissue Organ Cult 3:301–311
Zou YN (2010) Micropropagation of Chinese plum (Prunus salicina Lindl) using mature stem segments. Not Bot Horti Agrobot 38:214–218
Acknowledgments
This work was supported financially by a Fred M. van Eck scholarship for Purdue University to Ying Wang. The authors gratefully acknowledge Drs. Sadanand Dhekney, Lining Tian, and Charles Maynard for their constructive review and suggestions for the improvement of this manuscript. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the US Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that also may be suitable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Harold Trick
Rights and permissions
About this article
Cite this article
Wang, Y., Pijut, P.M. Improvement of Agrobacterium-mediated transformation and rooting of black cherry. In Vitro Cell.Dev.Biol.-Plant 50, 307–316 (2014). https://doi.org/10.1007/s11627-014-9608-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-014-9608-2