Abstract
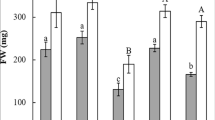
The effects of Ca2+ on antioxidative enzymes and indole-3-acetic acid (IAA) oxidase during adventitious rooting were investigated in mung bean (Vigna radiata). CaCl2 significantly promoted the formation and growth of adventitious roots. EGTA (a Ca2+ chelator) or ruthenium red (a Ca2+-channel blocker) significantly inhibited root formation and growth, but these inhibitory effects could be partially reversed by CaCl2. Furthermore, inclusion of 5 mM CaCl2 significantly increased superoxide dismutase (SOD) activity by 10% at 3 h and catalase (CAT) activity by an average of 29.6% at each time point. CaCl2 decreased peroxidase (POD) activity by 9.4% and 21% at 12 and 24 h, respectively, and ascorbate peroxidase (APX) activity by an average of 13.9% at each time point. These CaCl2-induced changes in enzymatic activities were similar to changes caused by indole-3-butyric acid (IBA). Treatment with EGTA or ruthenium red decreased SOD activity by an average of 18.4% and 15.2%, respectively; POD activity by 27.4% and 57.6%, respectively; APX activity by 10.3% and 15.6%, respectively; and CAT activity by 19.3% and 5.2%, respectively, when compared with CaCl2. In addition, CaCl2 increased IAA oxidase activity by an average of 5.5% beginning at 6 h, whereas EGTA significantly decreased IAA oxidase activity by 29.2%, 22.9%, and 13.5% at 6, 9, and 12 h, respectively. The inhibitory effects of EGTA could be partially suppressed by addition of CaCl2. These results imply that the stimulative effect of Ca2+ on adventitious rooting is partially related to Ca2+-induced changes in the activities of antioxidative enzymes and IAA oxidase.





Similar content being viewed by others
References
Agarwal S.; Sairam R. K.; Srivastava G. C.; Tyagi A.; Meena R. C. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidative enzymes induction in wheat seedlings. Plant Sci. 169: 559–570; 2005.
Batish D. R.; Singh H. P.; Kaur S.; Kohli R. K.; Yadav S. S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 165: 297–305; 2008.
Bellamine J.; Penel C.; Greppin H.; Gaspar T. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul. 26: 191–194; 1998.
Chao I. L.; Cho C. L.; Chen L. M.; Liu Z. H. Effect of indole-3-butyric acid on the endogenous indole-3-acetic acid and lignin contents in soybean hypocotyl during adventitious root formation. J. Plant Physiol. 158: 1257–1262; 2001.
Chen L. M.; Cheng J. T.; Chen E. L.; Yiu T. J.; Liu Z. H. Naphthaleneacetic acid suppresses peroxidase activity during the induction of adventitious roots in soybean hypocotyls. J. Plant Physiol. 159: 1349–1354; 2002.
De Gara L.; Paciolla C.; De Tullio M. C.; Motto M.; Arrigoni O. Ascorbate dependent hydrogen peroxide detoxification and ascorbate regeneration during germination of a highly productive maize hybrid: evidence of an improved detoxification mechanism against reactive oxygen species. Physiol. Plant. 109: 7–13; 2000.
De Klerk G. J.; Krieken W. V. D.; Jong J. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant 35: 189–199; 1999.
Falasca G.; Zaghi D.; Possenti M.; Altamura M. M. Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep. 23: 17–25; 2004.
Gazaryan I. G.; Chubar T. A.; Mareeva E. A.; Lagrimini L. M.; Vanhuystee R. B.; Thorneley R. N. F. Aerobic oxidation of indole-3-acetic acid catalyzed by anionic and cationic peanut peroxidase. Phytochemistry 51: 175–186; 1999.
Gong M.; Li Y. J.; Dai X.; Tian M.; Li Z. G. Involvement of calcium and calmodulin in the acquisition of HS induced thermotolerance in maize seedlings. J. Plant Physiol. 150: 615–621; 1997.
Hatzilazarou S. P.; Syros T. D.; Yupsanis T. A.; Bosabalidis A. M.; Economou A. S. Peroxidases, lignin and anatomy during in vitro and ex vitro rooting of gardenia (Gardenia jasminoides Ellis) microshoots. J. Plant Physiol. 163: 827–836; 2006.
Hepler P. K. Calcium: a central regulator of plant growth and development. Plant Cell 17: 2142–2155; 2005.
Hu X.; Jiang M.; Zhang J.; Zhang A.; Lin F.; Tan M. Calcium–calmodulin is required for abscisic acid-induced antioxidative defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol. 173: 27–38; 2007.
Li S. W.; Xue L. The interaction between H2O2 and NO, Ca2+, cGMP, and MAPKs during adventitious rooting in mung bean seedlings. In Vitro Cell. Dev. Biol. Plant 46: 142–148; 2010.
Li S. W.; Xue L.; Xu S.; Feng H.; An L. IBA-induced changes in antioxidative enzymes during adventitious rooting in mung bean seedlings: the role of H2O2. Environ. Exp. Bot. 66: 442–450; 2009.
Mato M. C.; Rua M. L.; Ferro E. Changes in levels of peroxidases and phenolics during root formation in Vitis cultured in vitro. Physiol. Plant. 72: 84–88; 1988.
Metaxas D.; Syros T.; Yupsanis T.; Economou A. S. Peroxidases during adventitious rooting in cuttings of Arbutus unedo and Taxus baccata as affected by plant genotype and growth regulator treatment. Plant Growth Regul. 44: 257–266; 2004.
Nag S.; Saha K.; Choudhuri M. A. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J. Plant Growth Regul. 20: 182–194; 2001.
Pei Z. M.; Murata Y.; Benning G.; Thomine S.; Klusener B.; Allen G. T.; Grill E.; Schroeder J. I. Calcium channels activated by hydrogen peroxide mediate abscisic signaling in guard cells. Nature 406: 731–734; 2000.
Pinto M. C.; Tommasi F.; De Gara L. Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow 2. Plant Physiol. Biochem. 38: 541–550; 2000.
Polle A.; Otter T.; Seifert F. Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol. 106: 53–60; 1994.
Pythoud F.; Buchala A. J. Peroxidase activity and adventitious rooting in cuttings of Populus tremula L. Plant Physiol. Biochem. 27: 503–510; 1989.
Quiroga M.; Guerrero C.; Botella M. A.; Barceló A.; Amaya I.; Medina M. I.; Alonso F. J.; Forchetti S. M.; Tigier H.; Valpuesta V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 122: 1119–1127; 2000.
Racchi M. L.; Bagnoli F.; Balla I.; Danti S. Differential activity of catalase and superoxide dismutase in seedlings and in vitro micropropagated oak (Quercus robur L.). Plant Cell Rep. 20: 169–174; 2001.
Rao M. V.; Paliyath G.; Ormrod D. P.; Murr D. O.; Watkins C. B. Influence of salicylic acid on H2O2 production, oxidative, stress, and H2O2-metabolizing enzymes. Plant Physiol. 115: 137–149; 1997.
Rout G. R. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regul. 48: 111–117; 2006.
Rout G. R.; Samantaray S.; Das P. Root induction in microshoots of Simarouba glauca L. in vitro: peroxidase as a marker for rooting. Silvae Genet 48: 14–17; 1999.
Sato Y.; Sugiyama M.; Górecki R. J.; Fukuda H.; Komamine A. Interrelationship between lignin deposition and the activities of peroxidase isoenzymes in differentiating tracheary elements of Zinnia. Planta 189: 584–589; 1993.
Singh H. P.; Kaur S.; Batish D. R.; Kohli R. K. Caffeic acid inhibits in vitro rooting in mung bean [Vigna radiata (L.) Wilczek] hypocotyls by inducing oxidative stress. Plant Growth Regul 57: 21–30; 2009.
Sorin C.; Bussell J. D.; Camus I.; Ljung K.; Kowalczyk M.; Geiss G.; McKhann H.; Garcion C.; Vaucheret H.; Sandberg G.; Bellini C. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 17: 1343–1359; 2005.
Sorin C.; Negroni L.; Balliau T.; Corti H.; Jacquemot M. P.; Davanture M.; Sandberg G.; Zivy M.; Bellini C. Proteomic analysis of different mutant genotypes of Arabidopsis led to the identification of 11 proteins correlating with adventitious root development. Plant Physiol. 140: 349–364; 2006.
Spychalla J. P.; Desborough S. L. Superoxide dismutase, catalase and alpha tocopherol content of stored potato tubers. Plant Physiol. 94: 1214–1218; 1990.
Syros T.; Yupsanis T.; Zafiriadis H.; Economou A. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 161: 69–77; 2004.
Toyota M.; Furuichi T.; Tatsumi H.; Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 146: 505–514; 2008.
Tyburski J.; Jasionowicz P.; Tretyn A. The effects of ascorbate on root regeneration in seedling cuttings of tomato. Plant Growth Regul. 48: 157–173; 2006.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31260090 and 30960063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Finer
Rights and permissions
About this article
Cite this article
Li, SW., Feng, L. & Zeng, XY. Effects of calcium on the activities of cytosolic antioxidative enzymes and IAA oxidase during in vitro adventitious rooting of mung bean seedlings. In Vitro Cell.Dev.Biol.-Plant 49, 750–758 (2013). https://doi.org/10.1007/s11627-013-9553-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9553-5