Abstract
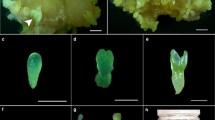
Soybean somatic embryos were developed as a model for investigating the developmental relationships of lipid biosynthesis and accumulation in this important crop. Batch cultures of embryos grown for 8 wk in liquid culture medium exhibited typical sigmoidal growth kinetics as they passed through characteristic globular, heart, torpedo, and cotyledon stages. Exponential growth occurred for the first 4 wk in culture with net growth terminating when total embryo fresh weight per culture flask reached a maximum of 4–4.5 g at 6 wk. This was followed by a slight decrease in embryo fresh weight (FW) and the onset of apparent tissue senescence as judged by yellowing and browning of embryos. On a FW basis, embryos accumulated up to 4% protein, 2.5% soluble sugars, 1.9% starch, and 1.5% lipid relatively early in development. Levels decreased to 0.8% protein, 0.5% soluble sugars, 0.03% starch, and 0.09% lipid at the end of the culture period. On a mass percent basis, lipid extracts were comprised of approximately 80–90% polar lipid early in embryo development. This shifted to 56% storage lipid (triacylglycerol) and 44% polar lipid after 4–5 wk in culture and then reverted back to 91% and 9% polar vs. storage lipid, respectively, by the end of the 8-wk culture period. On the average, polar and storage lipid fractions were comprised of 24% palmitic acid, 7% stearic acid, 8% oleic acid, 36% linoleic acid, and 26% linolenic acid. However, the amounts of linoleic and linolenic acids declined sharply during embryo senescence at the end of the culture period, with corresponding increases in the combined amounts of palmitic and stearic acids. This is the first report that documents the progress of storage reserve accumulation in soybean somatic embryos in relation to their continuous growth in liquid batch cultures.






Similar content being viewed by others
References
Adams C. A.; Rinne R. W.; Fjerstad M. C. Starch deposition and carbohydrase activities in developing and germinating soya bean seeds. Ann. Bot. 45: 577–582; 1980.
Bailey M. A.; Boerma H. R.; Parrott W. A. Genotype-specific optimization of plant regeneration from somatic embryos of soybean. Plant Sci. 93: 117–120; 1993.
Buchhein J. A.; Colburn S. M.; Ranch J. P. Maturation of soybean somatic embryos and the transition to plantlet growth. Plant Physiol. 89: 768–775; 1989.
Chanprame S.; Kuo T. M.; Widholm J. M. Soluble carbohydrate content of soybean [Glycine max (L.) Merr.] somatic and zygotic embryos during development. Vitro Cell Dev. Biol. Plant 34: 64–68; 1998.
Christou P.; Yang N. S. Developmental aspects of sobean (Glycine max) somatic embryogenesis. Ann. Bot. 64: 225–234; 1989.
Crawford N. M.; Kahn M. L.; Leustek T.; Long S. R. Nitrogen and sulfur. In: Buchanan B. B.; Gruissem W.; Jones R. L. (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 786–849; 2000.
da Silva P. M. F. R.; Eastmond P. J.; Hill L. M.; Smith A. M.; Rawsthorne S. Starch metabolism in developing embryos of oilseed rape. Planta 203: 480–487; 1997.
Dahmer M. L.; Collins G. B.; Hildebrand D. F. Lipid content and composition of soybean somatic embryos. Crop. Sci. 31: 741–746; 1991.
Dahmer M. L.; Hildebrand D. F.; Collins G. B. Comparative protein accumulation patterns in soybean somatic and zygotic embryos. Vitro Cell Dev. Biol. 28P: 106–114; 1992.
Eastmond P. J.; Rawsthorne S. Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryos. Plant Physiol. 122: 767–774; 2000.
Finer J. J.; Nagasawa A. Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tissue Organ Cult. 15: 126–136; 1988.
Gamborg O. L.; Miller R. A.; Ojima K. Nutrient requirement of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158; 1968.
Jamieson G. R.; Reid E. H. The occurrence of hexadeca-7,10,13-trienoic acid in the leaf lipids of angiosperms. Phytochemistry 10: 1837–1843; 1971.
Kinney A. J.; Clemente T. E. Modifying soybean oil for enhanced performance in biodiesel blends. Fuel Process. Tech. 86: 1137–1147; 2005.
Lazzeri P. A.; Hildebrand D. F.; Collins G. B. A procedure for plant regeneration from immature cotyledon tissue of soybean. Plant Mol. Biol. Rep. 3: 129–135; 1985.
Lippmann B.; Lippmann G. Induction of somatic embryos in cotyledonary tissue of soybean Glycine max L. Merr. Plant Cell Rep. 3: 215–218; 1984.
Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497; 1962.
Norton G.; Harris J. F. Compositional changes in developing rape seed (Brassica napus L.). Planta 123: 163–174; 1975.
Orzechowski S. Starch metabolism in leaves. Acta Biochim. Pol. 55: 435–455; 2008.
Parrott W. A.; Clemente T. E. Transgenic soybean. In: Specht J. E.; Boerma H. R. (eds) Soybeans: improvement, production, and uses. Agronomy monograph no. 16. 3rd ed. ASA-CSA-SSSA, Madison, pp 265–302; 2004.
Parrott W. A.; Dryden G.; Vogt S.; Hildebrand D. F.; Collins G. B.; Williams E. G. Optimization of somatic embryogenesis and embryo germination in soybean. Vitro Cell Dev. Biol. 24: 817–820; 1988.
Ranch J. P.; Oglesby L.; Zielinski A. C. Plant regeneration from embryo-derived tissue cultures of soybeans. Vitro Cell Dev. Biol. 21: 653–658; 1985.
Rawsthorne S. Carbon flux and fatty acid synthesis in plants. Progr. Lipid Res. 41: 182–196; 2002.
Samoylov V. M.; Tucker D. M.; Thibaud-Nissen F.; Parrott W. A. A liquid-medium-based protocol for rapid regeneration from embryogenic soybean cultures. Plant Cell Rep. 18: 49–54; 1998.
Schmidt M. A.; Tucker D. M.; Cahoon E. B.; Parrott W. A. Towards normalization of soybean somatic embryo maturation. Plant Cell Rep. 24: 383–391; 2005.
Shoemaker R. C.; Hammond E. G. Fatty acid composition of soybean (Glycine max (L.) Merr.) somatic embryos. Vitro Cell Dev. Biol. 24: 829–832; 1988.
Slawinska J.; Obendorf R. L. Soybean somatic embryo maturation: composition, respiration and water relations. Seed Sci. Res. 1: 251–262; 1991.
Smith A. M. Major differences in isoforms of starch-branching enzyme between developing embryos of round- and wrinkled-seeded peas (Pisum sativum L.). Planta 175: 270–279; 1988.
Tetlow I. J.; Rawsthorne S.; Raines C.; Emes M. J. Plastid metabolic pathways. In: Møller S. G. (ed) Plastids. CRC, Boca Raton, pp 60–125; 2005.
Thompson J. E.; Froese C. D.; Madey E.; Smith M. D.; Hong Y. Lipid metabolism during plant senescence. Progr. Lipid Res. 37: 119–141; 1998.
White C. A.; Kennedy J. F. Oligosaccharides. In: Chaplin M. F.; Kennedy J. F. (eds) Carbohydrate analysis—a practical approach. IRL Press, Oxford, pp 37–54; 1986.
Wilson R. F. Seed composition. In: Specht J. E.; Boerma H. R. (eds) Soybeans: improvement, production, and uses. Agronomy monograph no. 16. 3rd ed. ASA-CSA-SSSA, Madison, pp 621–677; 2004.
Yang Z.; Ohlrogge J. B. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium and switchgrass and in Arabidopsis beta-oxidation mutants. Plant Physiol. 150: 1981–1989; 2009.
Yazdi-Samadi B.; Rinne R. W.; Seif R. D. Components of developing soybean seeds: oil, protein, sugars, starch, organic acids, and amino acids. Agron. J. 69: 481–486; 1977.
Acknowledgments
The authors gratefully acknowledge the expert and generous technical assistance provided by Wayne Parrott and Donna Tucker in the Department of Crop and Soil Sciences at the University of Georgia in helping us implement their method for the in vitro growth of soybean somatic embryos. We are also grateful to Ian Stocks in the Department of Entomology, Soils and Plant Sciences at Clemson University for his initial help with the photomicrography equipment used in this investigation. This research was supported by Project no. 8233 provided to S. A. Sparace from the United Soybean Board.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John Finer
Rights and permissions
About this article
Cite this article
He, Y., Young, T.E., Clark, K.R. et al. Developmental profile of storage reserve accumulation in soybean somatic embryos. In Vitro Cell.Dev.Biol.-Plant 47, 725–733 (2011). https://doi.org/10.1007/s11627-011-9375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-011-9375-2