Abstract
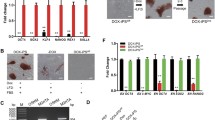
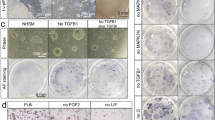
Porcine pluripotent stem cells had been derived from different culture systems. PeNK6 is a porcine pluripotent stem cell line that we established from an E5.5 embryo in a defined culture system. Signaling pathways related with pluripotency had been assessed in this cell line, and TGF-β signaling pathway–related genes were found upregulated significantly. In this study, we elucidated the role of the TGF-β signaling pathway in PeNK6 through adding small molecule inhibitors, SB431542 (KOSB) or A83-01 (KOA), into the original culture medium (KO) and analyzing the expression and activity of key factors involved in the TGF-β signaling pathway. In KOSB/KOA medium, the morphology of PeNK6 became compact and the nuclear-to-cytoplasm ratio was increased. The expression of the core transcription factor SOX2 was significantly upregulated compared with cell lines in the control KO medium, and the differentiation potential became balanced among three germ layers rather than bias to neuroectoderm/endoderm as the original PeNK6 did. The results indicated that inhibition of TGF-β has positive effects on the porcine pluripotency. Based on these results, we established a pluripotent cell line (PeWKSB) from E5.5 blastocyst by employing TGF-β inhibitors, and the cell line showed improved pluripotency.




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the manuscript, as well as from the corresponding author upon reasonable request.
References
Alberio R, Croxall N, Allegrucci C (2010) Pig epiblast stem cells depend on Activin/Nodal signaling for pluripotency and self-renewal. Stem Cells Dev 19:1627–1636
Boroviak T, Loos R, Bertone P, Smith A, Nichols J (2014) The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat Cell Biol 16:516–528
Brevini T, Pennarossa G, Maffei S, Gandolfi F (2012) Pluripotency network in porcine embryos and derived cell lines. Reprod Domest Anim 47(Suppl 4):86–91
Brevini TA, Tosetti V, Crestan M, Antonini S, Gandolfi F (2007) Derivation and characterization of pluripotent cell lines from pig embryos of different origins. Theriogenology 67:54–63
Burridge PW, Anderson D, Priddle H, Barbadillo Munoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C (2007) Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells 25:929–938
Deluz C, Friman ET, Strebinger D, Benke A, Raccaud M, Callegari A, Leleu M, Manley S, Suter DM (2016) A role for mitotic bookmarking of SOX2 in pluripotency and differentiation. Gene Dev 30:2538–2550
Du J, Wu Y, Ai Z, Shi X, Chen L, Guo Z (2014) Mechanism of SB431542 in inhibiting mouse embryonic stem cell differentiation. Cell Signal 26:2107–2116
Evans PM, Liu C (2008) Roles of Krüppel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim Biophys Sin (Shanghai) 40:554–564
Gao X, Nowak-Imialek M, Chen X, Chen D, Herrmann D, Ruan D, Chen ACH, Eckersley-Maslin MA, Ahmad S, Lee YL, Kobayashi T, Ryan D, Zhong J, Zhu J, Wu J, Lan G, Petkov S, Yang J, Antunes L, Campos LS, Fu B, Wang S, Yong Y, Wang X, Xue SG, Ge L, Liu Z, Huang Y, Nie T, Li P, Wu D, Pei D, Zhang Y, Lu L, Yang F, Kimber SJ, Reik W, Zou X, Shang Z, Lai L, Surani A, Tam PPL, Ahmed A, Yeung WSB, Teichmann SA, Niemann H, Liu P (2019) Establishment of porcine and human expanded potential stem cells. Nat Cell Biol 21:687–699
Ghimire S, Jeught M, Neupane J, Roost MS, Anckaert J, Popovic M, Nieuwerburgh FV, Mestdagh P, Vandesompele J, Deforce D (2018) Comparative analysis of naive, primed and ground state pluripotency in mouse embryonic stem cells originating from the same genetic background. Sci Rep 8(1):5884
Grace A, McMillan M, Schmoelzl S, Hinch G (2014) 187 increased efficiency of deriving bovine stem cell-like colonies using valproic acid and small-molecule cocktails. Reprod Fert Dev 26:208–208
Grannas K, Arngården L, Lönn P, Mazurkiewicz M, Blokzijl A, Zieba A, Söderberg O (2015) Crosstalk between Hippo and TGFβ: subcellular localization of YAP/TAZ/Smad complexes. J Mol Biol 427:3407–3415
Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A (2009) Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136:1063–1069
Hassani SN, Totonchi M, Sharifi-Zarchi A, Mollamohammadi S, Pakzad M, Moradi S, Samadian A, Masoudi N, Mirshahvaladi S, Farrokhi A (2014) Inhibition of TGFβ signaling promotes ground state pluripotency. Stem Cell Rev 10:16–30
Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74
Lee KL, Lim SK, Orlov YL, le Yit Y, Yang H, Ang LT, Poellinger L, Lim B (2011) Graded Nodal/Activin signaling titrates conversion of quantitative phospho-Smad2 levels into qualitative embryonic stem cell fate decisions. PLoS Genet 7:e1002130
Li M, Li L, Verma V, Liu Q, Shi D, Huang B, Zhang J (2015) An insight on small molecule induced foot-print free naive pluripotent stem cells in livestock. Stem Cell Discovery 5:1–9
Liu S, Bou G, Sun R, Guo S, Xue B, Wei R, Cooney AJ, Liu Z (2015) Sox2 is the faithful marker for pluripotency in pig: evidence from embryonic studies. Dev Dynam 244:619–627
Mahmood A, Harkness L, Schrøder HD, Abdallah BM, Kassem M (2010) Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542. J Bone Miner Res 25:1216–1233
Marcela GA, Leong LK, Mavrakis KJ, Paraskevi G, Norris DP, Vasso E, Mccabe BD (2009) Graded Smad2/3 activation is converted directly into levels of target gene expression in embryonic stem cells. PLoS One 4:e4268
Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA (2007) Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 9:625–635
Mullen AC, Wrana JL (2017) TGF-β family signaling in embryonic and somatic stem-cell renewal and differentiation. Csh Perspect Biol 9:a022186
Nagy K, Sung HK, Zhang P, Laflamme S, Vincent P, Agha-Mohammadi S, Woltjen K, Monetti C, Michael IP, Smith LC, Nagy A (2011) Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev Rep 7:693–702
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4:487–492
Ogawa K, Saito A, Matsui H, Suzuki H, Ohtsuka S, Shimosato D, Morishita Y, Watabe T, Niwa H, Miyazono K (2007) Activin-Nodal signaling is involved in propagation of mouse embryonic stem cells. J Cell Sci 120:55–65
Qi C, Zhang J, Wang Y, Lin M, Gao J, Lu H (2022) Valproic acid enhances neurosphere formation in cultured rat embryonic cortical cells through TGFβ1 signaling. J Biomed Res 36:127–140
Ramos-Ibeas P, Sang F, Zhu Q, Tang WWC, Withey S, Klisch D, Wood L, Loose M, Surani MA, Alberio R (2019) Pluripotency and X chromosome dynamics revealed in pig pre-gastrulating embryos by single cell analysis. Nat Commun 10:500
Secher JO, Callesen H, Freude KK, Hyttel P (2016) Initial embryology and pluripotent stem cells in the pig–the quest for establishing the pig as a model for cell therapy. Theriogenology 85:162–171
Shariatinia Z, Mazloom-Jalali A (2019) Chitosan nanocomposite drug delivery systems designed for the ifosfamide anticancer drug using molecular dynamics simulations. J Mol Liq 273:346–367
Sharma R, Kamble N, George A, Chauhan M, Singla S, Manik R, Palta P (2013) Effect of TGF-β1 superfamily members on survival of buffalo (Bubalus bubalis) embryonic stem-like cells. Reprod Domest Anim 48:569–576
Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA (2008) Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol 313:107–117
Talbot NC, Le Blomberg A (2008) The pursuit of ES cell lines of domesticated ungulates. Stem Cell Rev 4:235–254
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199
Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M, Imamura T (2010) The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci 96:791
Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA (2009) Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development 136:1339–1349
Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, Sasaki H, Ralston A (2014) HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genet 10(10):e1004618
Xue B, Li Y, He Y, Wei R, Liu Z (2016) Porcine pluripotent stem cells derived from IVF embryos contribute to chimeric development in vivo. PLoS ONE 11:e0151737
Yang F, Wang N, Wang Y, Yu T, Wang H (2017) Activin-SMAD signaling is required for maintenance of porcine iPS cell self-renewal through upregulation of NANOG and OCT4 expression. J Cell Physiol 232:2253–2262
Ye S, Li P, Tong C, Ying QL (2014) Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. Embo J 32:2548–2560
Zhang S, Cui W (2014) Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells 6:305
Zhang X, Xue B, Li Y, Wei R, Yu Z, Jin J, Zhang Y, Liu Z (2019) A novel chemically defined serum- and feeder-free medium for undifferentiated growth of porcine pluripotent stem cells. J Cell Physiol 234:15380–15394
Acknowledgements
We thank Renyue Wei, Jiawei Lv, and Jiaqiang Wang for their technical assistance. This work was supported by the National Key Research and Development program of China-Stem cell and Translational Research (2016YFA0100200).
Author information
Authors and Affiliations
Contributions
Fang Gao and Shuang Wu conceived the study, designed and carried out the experiments, and drafted the manuscript; Yan Li, Yuan Fang, Minli Liu, Jiawei Du, and Qingran Kong carried out the experiments and participated in data analysis; Tiezhu An supervised the study. All authors have read, discussed, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All animal experiments were approved by the Animal Care and Use Committee of Northeast Forestry University and the Animal Care and Use Committee of Northeast Agricultural University and were performed according to the guidelines for the welfare and use of animals in stem cell research.
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, F., Wu, S., Li, Y. et al. Inhibition of TGF-β pathway improved the pluripotency of porcine pluripotent stem cells. In Vitro Cell.Dev.Biol.-Animal 59, 142–152 (2023). https://doi.org/10.1007/s11626-023-00752-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00752-8