Abstract
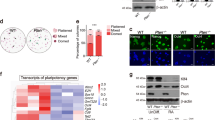
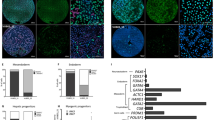
Embryonic stem (ES) cells are considered to exist in a ground state if shielded from differentiation triggers. Here we show that FGF4 and TGFβ signaling pathway inhibitors, designated R2i, not only provide the ground state pluripotency in production and maintenance of naïve ES cells from blastocysts of different mouse strains, but also maintain ES cells with higher genomic integrity following long-term cultivation compared with the chemical inhibition of the FGF4 and GSK3 pathways, known as 2i. Global transcriptome analysis of the ES cells highlights augmented BMP4 signaling pathway. The crucial role of the BMP4 pathway in maintaining the R2i ground state pluripotency is demonstrated by BMP4 receptor suppression, resulting in differentiation and cell death. In conclusion, by inhibiting TGFβ and FGF signaling pathways, we introduce a novel defined approach to efficiently establish the ground state pluripotency.






Similar content being viewed by others
Change history
06 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12015-021-10268-x
References
Evans, M. J., & Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156.
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America, 78(12), 7634–7638.
Smith, A. G., et al. (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature, 336(6200), 688–690.
Ying, Q. L., et al. (2003). BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell, 115(3), 281–292.
Matsuda, T., et al. (1999). STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO Journal, 18(15), 4261–4269.
Niwa, H., et al. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes & Development, 12(13), 2048–2060.
Wray, J., Kalkan, T., & Smith, A. G. (2010). The ground state of pluripotency. Biochemical Society Transactions, 38(4), 1027–1032.
Ying, Q. L., et al. (2008). The ground state of embryonic stem cell self-renewal. Nature, 453(7194), 519–523.
Martello, G., et al. (2012). Esrrb is a pivotal target of the gsk3/tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell, 11(4), 491–504.
Valvezan, A. J., et al. (2012). Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. Journal of Biological Chemistry, 287(6), 3823–3832.
Acevedo, N., et al. (2007). Glycogen synthase kinase-3 regulation of chromatin segregation and cytokinesis in mouse preimplantation embryos. Molecular Reproduction and Development, 74(2), 178–188.
Tighe, A., et al. (2007). GSK-3 inhibitors induce chromosome instability. BMC Cell Biology, 8, 34.
Hassani, S. N., et al. (2012). Simultaneous suppression of TGF-beta and ERK signaling contributes to the highly efficient and reproducible generation of mouse embryonic stem cells from previously considered refractory and non-permissive strains. Stem Cell Reviews, 8(2), 472–481.
Baharvand, H., & Hassani, S. N. (2013). A new chemical approach to the efficient generation of mouse embryonic stem cells. Methods in Molecular Biology, 997, 13–22.
Ritchie, M. E., et al. (2007). A comparison of background correction methods for two-colour microarrays. Bioinformatics, 23(20), 2700–2707.
Smyth, G., Thorne, N., & Wettenhall J., LIMMA: Linear Models for Microarray Data User’s Guide, 2003. URL http://www.bioconductor.org.
R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org.
Baharvand, H., & Matthaei, K. I. (2004). Culture condition difference for establishment of new embryonic stem cell lines from the C57BL/6 and BALB/c mouse strains. In Vitro Cellular & Developmental Biology. Animal, 40(3–4), 76–81.
Kress, C., et al. (1998). Nonpermissiveness for mouse embryonic stem (ES) cell derivation circumvented by a single backcross to 129/Sv strain: establishment of ES cell lines bearing the Omd conditional lethal mutation. Mammalian Genome, 9(12), 998–1001.
Blair, K., Wray, J., & Smith, A. (2011). The liberation of embryonic stem cells. PLoS Genetics, 7(4), e1002019.
Marks, H., et al. (2012). The transcriptional and epigenomic foundations of ground state pluripotency. Cell, 149(3), 590–604.
Hamada, H., et al. (2002). Establishment of vertebrate left-right asymmetry. Nature Reviews Genetics, 3(2), 103–113.
Rebuzzini, P., et al. (2008). Karyotype analysis of the euploid cell population of a mouse embryonic stem cell line revealed a high incidence of chromosome abnormalities that varied during culture. Cytogenetic and Genome Research, 121(1), 18–24.
Wong, E. S., et al. (2010). A simple procedure for the efficient derivation of mouse ES cells. Methods in Enzymology, 476, 265–283.
Xu, R. H., et al. (2008). NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell, 3(2), 196–206.
Fei, T., et al. (2010). Smad2 mediates Activin/Nodal signaling in mesendoderm differentiation of mouse embryonic stem cells. Cell Research, 20(12), 1306–1318.
Ogawa, K., et al. (2007). Activin-Nodal signaling is involved in propagation of mouse embryonic stem cells. Journal of Cell Science, 120(Pt 1), 55–65.
Watabe, T., & Miyazono, K. (2009). Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Research, 19(1), 103–115.
Heldin, C. H., Miyazono, K., & ten Dijke, P. (1997). TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature, 390(6659), 465–471.
Akhurst, R. J., et al. (1990). TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development, 108(4), 645–656.
Zwijsen, A., et al. (1999). Ectopic expression of the transforming growth factor beta type II receptor disrupts mesoderm organisation during mouse gastrulation. Developmental Dynamics, 214(2), 141–151.
Lee, K. L., et al. (2011). Graded Nodal/Activin signaling titrates conversion of quantitative phospho-Smad2 levels into qualitative embryonic stem cell fate decisions. PLoS Genetics, 7(6), e1002130.
Mullen, A. C., et al. (2011). Master transcription factors determine cell-type-specific responses to TGF-beta signaling. Cell, 147(3), 565–576.
James, D., et al. (2005). TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development, 132(6), 1273–1282.
Ichida, J. K., et al. (2009). A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell, 5(5), 491–503.
Maherali, N., & Hochedlinger, K. (2009). Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Current Biology, 19(20), 1718–1723.
Li, Z., et al. (2012). BMP4 Signaling Acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell, 10(2), 171–182.
Loh, K. M., & Lim, B. (2011). A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell, 8(4), 363–369.
Acknowledgments
We thank the members of the Department of Stem Cells and Developmental Biology labs for their helpful suggestions and critical reading of the manuscript. We thank Behrouz Asgari for chimera formation and germline transmission. This study was funded by grants provided from Royan Institute and Iranian Council of Stem Cell Technology and the Iran National Science Foundation (INSF).
Author Contributions
S. H., M. T., H. R. S. and H. B. designed all experiments and wrote the manuscript. S. H. and S. M. performed cell culture. M. T. and A. S. performed real-time PCR analysis. A. F. and M. P. operated in vivo experiments. N. M. and H. G. performed karyotype analysis. S. M. and G. S. H. performed western blot analysis. M. T., A. S., M. S., B. G. and M. J. A. designed and interpreted microarray analysis. G. H. S., S. M. and D. S. contributed to the overall design and writing of the article.
Conflict of Interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Seyedeh-Nafiseh Hassani and Mehdi Totonchi contributed equally in this work.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 9.11 MB)
Rights and permissions
About this article
Cite this article
Hassani, SN., Totonchi, M., Sharifi-Zarchi, A. et al. Inhibition of TGFβ Signaling Promotes Ground State Pluripotency. Stem Cell Rev and Rep 10, 16–30 (2014). https://doi.org/10.1007/s12015-013-9473-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-013-9473-0