Abstract
Introduction
C-reactive protein may predict anastomotic complications after colorectal surgery, but its predictive ability may differ between laparoscopic and open resection due to differences in stress response. Therefore, the objective of this study was to perform a systematic review and meta-analysis on the diagnostic characteristics of C-reactive protein to detect anastomotic leaks and infectious complications after laparoscopic and open colorectal surgery.
Methods
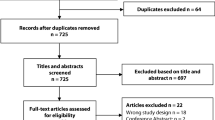
A systematic review was performed according to PRISMA. Studies were included if they reported on the diagnostic characteristics of postoperative day 3–5 values of serum C-reactive protein to diagnose anastomotic leak or infectious complications specifically in patients undergoing elective laparoscopic and open colorectal surgery. The main outcome was a composite of anastomotic leak and infectious complications. A random-effects model was used to perform a meta-analysis of diagnostic accuracy.
Results
A total of 13 studies were included (9 for laparoscopic surgery, 8 for open surgery). The pooled incidence of the composite outcome was 14.8% (95% CI 10.2–19.3) in laparoscopic studies and 21.0% (95% CI 11.9–30.0) for open. The pooled diagnostic accuracy characteristics were similar for open and laparoscopic studies. However, the C-reactive protein threshold cutoffs were lower in laparoscopic studies for postoperative days 3 and 4, but similar on day 5.
Conclusions
The diagnostic characteristics of C-reactive protein in the early postoperative period to detect infectious complications and leaks are similar after laparoscopic and open colorectal surgery. However, thresholds are lower for laparoscopic surgery, suggesting that the interpretation of serum CRP values needs to be tailored based on operative approach.




Similar content being viewed by others
References
Midura EF, Hanseman D, Davis BR, Atkinson SJ, Abbott DE, Shah SA et al. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum. 2015;58(3):333-8. https://doi.org/10.1097/DCR.0000000000000249.
Tevis SE, Carchman EH, Foley EF, Heise CP, Harms BA, Kennedy GD. Does Anastomotic Leak Contribute to High Failure-to-rescue Rates? Ann Surg. 2016;263(6):1148-51. https://doi.org/10.1097/SLA.0000000000001409.
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(5):890-9. https://doi.org/10.1097/SLA.0b013e3182128929.
Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261(3):497-505. https://doi.org/10.1097/SLA.0000000000000854.
Tan WJ, Ng WQ, Sultana R, de Souza NN, Chew MH, Foo FJ et al. Systematic review and meta-analysis of the use of serum procalcitonin levels to predict intra-abdominal infections after colorectal surgery. Int J Colorectal Dis. 2018;33(2):171-80. https://doi.org/10.1007/s00384-017-2956-8.
Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(4):339-46. https://doi.org/10.1002/bjs.9354.
Gans SL, Atema JJ, van Dieren S, Groot Koerkamp B, Boermeester MA. Diagnostic value of C-reactive protein to rule out infectious complications after major abdominal surgery: a systematic review and meta-analysis. Int J Colorectal Dis. 2015;30(7):861-73. https://doi.org/10.1007/s00384-015-2205-y.
Madbouly KM, Senagore AJ, Delaney CP. Endogenous morphine levels after laparoscopic versus open colectomy. Br J Surg. 2010;97(5):759-64. https://doi.org/10.1002/bjs.6987.
Kuntz C, Wunsch A, Bay F, Windeler J, Glaser F, Herfarth C. Prospective randomized study of stress and immune response after laparoscopic vs conventional colonic resection. Surg Endosc. 1998;12(7):963-7. https://doi.org/10.1007/s004649900757.
Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255(2):216-21. https://doi.org/10.1097/SLA.0b013e31824336e2.
Tsimogiannis KE, Tellis CC, Tselepis AD, Pappas-Gogos GK, Tsimoyiannis EC, Basdanis G. Toll-like receptors in the inflammatory response during open and laparoscopic colectomy for colorectal cancer. Surg Endosc. 2012;26(2):330-6. https://doi.org/10.1007/s00464-011-1871-2.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ journal of surgery. 2003;73(9):712-6.
Cole DS, Watts A, Scott-Coombes D, Avades T. Clinical utility of peri-operative C-reactive protein testing in general surgery. Ann R Coll Surg Engl. 2008;90(4):317-21. https://doi.org/10.1308/003588408X285865.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. https://doi.org/10.1136/bmj.d549.
Simundic AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC. 2009;19(4):203-11.
Linn S, Grunau PD. New patient-oriented summary measure of net total gain in certainty for dichotomous diagnostic tests. Epidemiol Perspect Innov. 2006;3:11. https://doi.org/10.1186/1742-5573-3-11.
Adamina M, Warschkow R, Naf F, Hummel B, Rduch T, Lange J et al. Monitoring c-reactive protein after laparoscopic colorectal surgery excludes infectious complications and allows for safe and early discharge. Surg Endosc. 2014;28(10):2939-48. https://doi.org/10.1007/s00464-014-3556-0.
Facy O, Paquette B, Orry D, Santucci N, Rat P, Rat P et al. Inflammatory markers as early predictors of infection after colorectal surgery: the same cut-off values in laparoscopy and laparotomy? Int J Colorectal Dis. 2017;32(6):857-63. https://doi.org/10.1007/s00384-017-2805-9.
Mik M, Dziki L, Berut M, Trzcinski R, Dziki A. Neutrophil to Lymphocyte Ratio and C-Reactive Protein as Two Predictive Tools of Anastomotic Leak in Colorectal Cancer Open Surgery. Dig Surg. 2018;35(1):77-84. https://doi.org/10.1159/000456081.
Munoz JL, Alvarez MO, Cuquerella V, Miranda E, Pico C, Flores R et al. Procalcitonin and C-reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program. Surg Endosc. 2018;32(9):4003-10. https://doi.org/10.1007/s00464-018-6144-x.
Nason GJ, Barry BD, Obinwa O, McMacken E, Rajaretnam NS, Neary PC. Early rise in C-reactive protein is a marker for infective complications in laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech. 2014;24(1):57-61. https://doi.org/10.1097/SLE.0b013e31828fa03e.
Pedersen T, Roikjaer O, Jess P. Increased levels of C-reactive protein and leukocyte count are poor predictors of anastomotic leakage following laparoscopic colorectal resection. Dan Med J. 2012;59(12):A4552.
Pedrazzani C, Moro M, Mantovani G, Lazzarini E, Conci S, Ruzzenente A et al. C-reactive protein as early predictor of complications after minimally invasive colorectal resection. J Surg Res. 2017;210:261-8. https://doi.org/10.1016/j.jss.2016.11.047.
Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG et al. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19(13):4168-77. https://doi.org/10.1245/s10434-012-2498-9.
Ramanathan ML, MacKay G, Platt J, Horgan PG, McMillan DC. The impact of open versus laparoscopic resection for colon cancer on C-reactive protein concentrations as a predictor of postoperative infective complications. Ann Surg Oncol. 2015;22(3):938-43. https://doi.org/10.1245/s10434-014-4065-z.
Ramos Fernandez M, Rivas Ruiz F, Fernandez Lopez A, Loinaz Segurola C, Fernandez Cabrian JM, de la Portilla de Juan F. C Reactive Protein as a Predictor of Anastomotic Leakage in Colorectal Surgery. Comparison Between Open and Laparoscopic Surgery. Cir Esp. 2017;95:529-35.
Welsch T, Muller SA, Ulrich A, Kischlat A, Hinz U, Kienle P et al. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int J Colorectal Dis. 2007;22(12):1499-507. https://doi.org/10.1007/s00384-007-0354-3.
Waterland P, Ng J, Jones A, Broadley G, Nicol D, Patel H et al. Using CRP to predict anastomotic leakage after open and laparoscopic colorectal surgery: is there a difference? Int J Colorectal Dis. 2016;31(4):861-8. https://doi.org/10.1007/s00384-016-2547-0.
Warschkow R, Tarantino I, Torzewski M, Naf F, Lange J, Steffen T. Diagnostic accuracy of C-reactive protein and white blood cell counts in the early detection of inflammatory complications after open resection of colorectal cancer: a retrospective study of 1,187 patients. Int J Colorectal Dis. 2011;26(11):1405-13. https://doi.org/10.1007/s00384-011-1262-0.
Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157(2):362-80. https://doi.org/10.1016/j.surg.2014.09.009.
Fink-Neuboeck N, Lindenmann J, Bajric S, Maier A, Riedl R, Weinberg AM et al. Clinical impact of interleukin 6 as a predictive biomarker in the early diagnosis of postoperative systemic inflammatory response syndrome after major thoracic surgery: A prospective clinical trial. Surgery. 2016;160(2):443-53. https://doi.org/10.1016/j.surg.2016.04.004.
McSorley ST, Khor BY, MacKay GJ, Horgan PG, McMillan DC. Examination of a CRP first approach for the detection of postoperative complications in patients undergoing surgery for colorectal cancer: A pragmatic study. Medicine (Baltimore). 2017;96(7):e6133. https://doi.org/10.1097/MD.0000000000006133.
Holl S, Fournel I, Orry D, Facy O, Cheynel N, Rat P et al. Should CT scan be performed when CRP is elevated after colorectal surgery? Results from the inflammatory markers after colorectal surgery study. Journal of visceral surgery. 2017;154(1):5-9. https://doi.org/10.1016/j.jviscsurg.2016.07.003.
Benedetti M, Ciano P, Pergolini I, Ciotti S, Guercioni G, Ruffo G et al. Early diagnosis of anastomotic leakage after colorectal surgery by the Dutch leakage score, serum procalcitonin and serum C-reactive protein: study protocol of a prospective multicentre observational study by the Italian ColoRectal Anastomotic Leakage (iC). G Chir. 2019;40(1):20-5.
Kornmann V, van Ramshorst B, van Dieren S, van Geloven N, Boermeester M, Boerma D. Early complication detection after colorectal surgery (CONDOR): study protocol for a prospective clinical diagnostic study. Int J Colorectal Dis. 2016;31(2):459-64. https://doi.org/10.1007/s00384-015-2468-3.
Barbic J, Ivic D, Alkhamis T, Drenjancevic D, Ivic J, Harsanji-Drenjancevic I et al. Kinetics of changes in serum concentrations of procalcitonin, interleukin-6, and C- reactive protein after elective abdominal surgery. Can it be used to detect postoperative complications? Coll Antropol. 2013;37(1):195-201.
Persec J, Persec Z, Husedzinovic I. Postoperative pain and systemic inflammatory stress response after preoperative analgesia with clonidine or levobupivacaine: a randomized controlled trial. Wien Klin Wochenschr. 2009;121(17-18):558-63. https://doi.org/10.1007/s00508-009-1221-8.
Cabellos Olivares M, Labalde Martinez M, Torralba M, Rodriguez Fraile JR, Atance Martinez JC. C-reactive protein as a marker of the surgical stress reduction within an ERAS protocol (Enhanced Recovery After Surgery) in colorectal surgery: A prospective cohort study. J Surg Oncol. 2018;117(4):717-24. https://doi.org/10.1002/jso.24909.
Author information
Authors and Affiliations
Contributions
Conception or design: JF, GMF, LSF, LL
Data acquisition, analysis, or interpretation of data: TP, AZ, MT, JF, GMF, LSF, LL
Manuscript drafting: TP, AZ, MT, LL
Critical revision: JF, GMF, LSF, LL
Final approval of the version to be published: TP, AZ, MT, JF, GMF, LSF, LL
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: TP, AZ, MT, JF, GMF, LSF, LL
Corresponding author
Ethics declarations
Conflict of Interest
LL is the recipient of an investigator-initiated grant from Johnson & Johnson. JF has received investigator-initiated grants from Merck and personal fees for consulting from Shionogi. LSF receives consulting fees from Merck and Abbott. TP, AZ, MT, and GMF have no conflicts of interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paradis, T., Zorigtbaatar, A., Trepanier, M. et al. Meta-analysis of the Diagnostic Accuracy of C-Reactive Protein for Infectious Complications in Laparoscopic Versus Open Colorectal Surgery. J Gastrointest Surg 24, 1392–1401 (2020). https://doi.org/10.1007/s11605-020-04599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04599-2