Abstract
Background and Aims
Intestinal stem cell organoid transplantation generates functional intestinal neomucosa and has been used therapeutically to improve nutrient absorption and cure bile acid malabsorption in rats. We hypothesized that intestinal organoids can be harvested and transplanted to generate intestinal neomucosa in a large animal model.
Materials and Methods
In group 1, 2-month old beagles (n = 6) underwent autotransplantation of intestinal organoids prepared from a segment of their own ileum. In group 2, intestinal organoids were harvested from fetuses and allotransplanted into 10-month old mother animals (n = 4). Tissues were harvested after 4 weeks and analyzed by hematoxylin and eosin histology and fluorescent microscopy.
Results
Large numbers of viable organoids were harvested in both groups. In group 1, no neomucosal growth was identified in any of the engraftment sites after autotransplantation of juvenile organoids. In group 2, neomucosal growth with large areas of crypts and villi was identified in 11 of 12 polyglycolic acid scaffolds after allotransplantation of fetal organoids. The neomucosa resembled normal canine mucosa in structure and composition.
Conclusions
Intestinal stem cell organoid transplantation can be used to generate neomucosa in dogs. This is the first report of successful generation of intestinal neomucosa using intestinal stem cell organoid transplantation in a large animal model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Massive loss of small bowel leads to a short bowel syndrome with significant morbidity, including malnutrition, diarrhea, electrolyte abnormalities, profound dehydration, and failure to thrive.1–3 Current therapeutic options for short bowel syndrome are limited. They include total parenteral nutrition, bowel lengthening procedures, and small bowel transplantation, all of which carry significant morbidity and mortality.4 Many patients with these conditions could be more effectively treated if healthy mucosa were available in larger quantities as a replacement or functional supplement. Hence, methods to generate neomucosa, for example, by transplanting intestinal mucosal stem cells, represent a potentially appealing alternative.
Intestinal stem cell “organoids” are multi-cellular aggregates of intestinal mucosal progenitors and putative mucosal stem cells, which surround a core of mesenchymal stromal cells.5 Organoids are the smallest transplantable unit of this mucosal tissue identified to date. Transplantation of intestinal organoids has been shown to generate intestinal neomucosa in rats that resembles native intestine in both structure and function.6–11 We have recently shown that transplantation of rat ileal organoids into debrided segments of jejunum generates a “neo-ileum” that completely reverses bile acid malabsorption in rats that have undergone ileal resection.10 In other studies, Grikscheit et al. anastomosed tissue-engineered mucosal cysts to the native intestine at the time of an 85% enterectomy in rats and showed that it reduced postoperative weight loss compared with animals that underwent only small bowel resection.11 Based on these studies, it is clear that intestinal organoid transplantation has potential therapeutic benefits.
In spite of these successful rodent studies, successful generation of neomucosa using intestinal organoid transplantation has never been reported in large animals. In the present study, we show that the isolation and transplantation of intestinal organoids can be used to generate neomucosa in a dog model.
Materials and Methods
Animals
Beagles were obtained from Marshall Farms (North Rose, NY, USA). Animals were allowed an acclimatization period of 1 week before participation in experiments in accordance with Institutional Animal Care and Use Committee guidelines. All animals were housed in accordance with the National Institutes of Health guidelines for the care of laboratory animals, maintained under a 12-hour light/dark cycle (6 a.m. to 6 p.m.) and received standard dog chow twice a day (Nestle Purina, St. Louis, MS, USA) and water ad libitum.
Study Design
Preliminary pilot experiments were performed on three 2-month-old beagles (P1, P2, and P3) to optimize the method of intestinal organoid isolation (Table 1). We aimed to obtain clusters that were microscopically similar to those produced with our previously successful rodent protocols.8,10,12,13
We then conducted two different types of experiments involving autotransplantation of organoids and allotransplantation of organoids, respectively. In experimental group 1, autotransplantation experiments were performed (Table 1). Two-month-old male beagles were used as both donors for intestinal stem cell isolation and as recipients for transplantation. The group consisted of six animals (n = 6), Auto-1, 2, 3, 4, 5, and 6. In experimental group 2, allotransplantation experiments were performed. Ten-month-old pregnant female beagles were used. The group consisted of four mother animals (n = 4), Allo-1, 2, 3, and 4. Their fetal pups (gestational days 40–50) were removed via cesarean section and used as the donors for the intestinal organoid isolation. The mother animals were used as non-syngeneic recipients of the intestinal organoids. All four animals had organoids seeded onto tubularized polyglycolic acid (PGA) biopolymers (Synthecon, Houston, TX, USA), which were wrapped in omentum (Table 1).
Surgery
Animals were fasted 24 h before surgery and kept nothing per os from the night before surgery. All pharmaceutical drugs were obtained from McKesson Pharmaceutical (San Francisco, CA, USA) unless otherwise noted. On the morning of the surgery, each animal was sedated with subcutaneously administered acepromazine (0.025 mg/kg; Butler Animal Health Supply, Dublin, OH, USA) 1 h before anesthesia. A transdermal fentanyl patch (25 μg/72 h) was placed on the dorsal skin for perioperative pain control. After intravenous administration of atropine (0.05 mg/kg; Butler Animal Health Supply, Dublin, OH, USA) and diazepam (0.275 mg/kg), the animal was endotracheally intubated and maintained on inhaled isoflurane (0.8–2.0% in oxygen; Butler Animal Health Supply, Dublin, OH, USA) anesthesia for the duration of the procedure. Cefazolin (20 mg/kg) was administered intravenously before making the skin incision. All suture materials were obtained from Ethicon (Sommerville, NJ, USA).
Group 1—Autotransplantation
To harvest ileal organoids, a midline laparotomy was made under sterile conditions. The distal 40 cm of ileum was resected and transferred to the laboratory for ileal organoid isolation. The proximal and distal resection sites were re-anastomosed to restore the continuity of the gastrointestinal tract. The animals were maintained under anesthesia while the organoid isolation was performed.
Isolation of Juvenile Organoids
Intestinal organoids were isolated using a modification of a technique described by Avansino et al.8 In brief, the 40-cm length of excised ileum was rinsed with 1 l of pre-warmed sterile 0.9% saline, followed by 4 l of pre-warmed polyethylene glycol (PEG) (5.9% in water) (Braintree Laboratories, Braintree, MA, USA) solution to remove the large amounts of mucus present in the juvenile canine intestine. The rinsed ileum was then opened longitudinally, and the mucosa was scraped off with a glass slide and minced into pieces. The tissue was transferred to a large tissue culture flask and washed three times in calcium- and magnesium-free Hanks’ buffered saline solution (HBSS*; Mediatech Inc., Herndon, VA, USA), with 100 IU/ml penicillin + 100 μg/ml streptomycin (Gibco, Gaitersburg, MD, USA) and 4 mM l-glutamine (Invitrogen, Carlsbad, CA, USA). A fourth wash included 2 mM N-acetyl cysteine (NAC; Abbott Laboratories, Chicago, IL, USA) as a mucolytic agent. The epithelial clumps were shaken gently for 10 min at room temperature. A final wash was performed in HBSS*. The tissue was minced into ≤1 mm pieces and transferred into an HBSS* solution containing 0.1 mg/ml dispase type 1 (Roche, Indianapolis, IN, USA) and 300 U/ml collagenase type XI (Sigma, St Louis, MO, USA) and shaken on the orbital incubator at 250 rpm at 37°C for 40 min. The suspension was transferred into HBSS* with 2 mM NAC and shaken at room temperature for 5 min. The contents were allowed to sediment for 1 min, and the upper mucous layer was removed and discarded. The supernatant was transferred into HBSS* and gently inverted and allowed to sediment for 2 min. The upper mucus layer was removed and discarded and the step repeated. Two parts of this cleared supernatant were mixed with one part of Dulbecco’s modified Eagle’s medium (Gibco, Gaitersburg, MD, USA) to which 2% d-sorbitol (Sigma, St Louis, MO, USA), 2.5% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), and 100 IU/ml penicillin + 100 μg/ml streptomycin (Gibco, Gaitersburg, MD, USA) had been added (DMEM-S). The mixture was centrifuged at 1,600 rpm for 4 min at room temperature, and the supernatant was decanted. The pellet was washed in DMEM-S six times. The intestinal organoids were then seeded directly or first labeled (see below) and then seeded.
Labeling of Intestinal Stem Cell Clusters with Fluorescent Cell Markers
Carboxyfluorescein diacetate succinimidyl ester (Vybrant ® CFDA SE) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Vybrant ® DiI) cell labeling solutions were obtained from Invitrogen (Carlsbad, CA, USA). The intestinal organoids were suspended at a density of 20,000 clusters per milliliter in DMEM-S and labeled for 10 min at 37°C following the manufacturer’s recommendations. The suspensions were centrifuged at 1,500 rpm for 5 min at 37°C and the supernatant discarded. The organoids were gently resuspended in 37°C DMEM-S and washed three times. The organoids were then resuspended in DMEM-S and seeded. An aliquot of organoids were left unlabeled to control for any possible adverse effect that the labeling process may have had on organoid viability. Organoid cell viability was tested with Trypan blue (Invitrogen, Carlsbad, CA, USA) exclusion.
Lentiviral Transduction of Organoids with DsRed
Intestinal organoids (20,000 clusters ~2 × 106 cells) were suspended in 600 μl of pre-warmed 37°C DMEM supplemented with 10% FBS, 100 IU/ml penicillin + 100 μg/ml streptomycin (Gibco, Gaitersburg, MD, USA), and diethylaminoethyl-dextran (DEAE-dextran; 16.7 μg/ml; Amersham Biosciences, Piscataway, NJ, USA). The DsRed lentiviral transfer vector RRLsin.cPPT.hPGK.DsRed.Wpre (a kind gift from Dr. Hans-Peter Kiem, University of Washington) expresses the fluorescent protein DsRed from the internal human phosphoglycerate kinase promoter containing a woodchuck hepatitis pre-element as well as a central polyurine tract.14 Freshly thawed and pre-warmed lentivirus in DMEM was added to the warm intestinal stem cell solution and gently inverted. The final solution volume had a final concentration of 1.08 × 106 IU of lentivirus per milliliter and 10 μg/ml of DEAE-dextran. The mixture was incubated at 37°C for 30 min with gentle inversion. The cells were then washed six times in DMEM (with 10% FBS and 100 IU/ml penicillin and 100 μg/ml streptomycin) to remove any remaining virus. The pellet was resuspended in DMEM and used for seeding. An aliquot of the transduced organoids were transferred into tissue culture for 48 h to confirm transduction efficiency.
Biopolymer Preparation and Sterilization
Non-woven sheets of PGA (2-mm thick, 95% void volume, 60 mg of PGA/ml, fiber diameter of 13 μm) were obtained from Synthecon (Houston, TX, USA). The PGA polymer sheets were tubularized into 1-cm long tubes with an internal radius of 8 mm using 6-0 Vicryl suture. The tubes were sterilized for 30 min in 80% ethanol and subsequently rinsed with 1.5 l of sterile phosphate buffered solution (PBS) (Fisher, Pittsburgh, PA). The tubes were placed into a 0.3% collagen type 1 solution in PBS (Vitrogen 100, Cohesion Tech, Palo Alto, CA, USA) for 30 min and then rinsed with PBS. They were finally vacuum-dried (Speed Vac, Savant Instruments, Farmingdale, NY, USA) for 30 min at 25°C and stored at 4°C under sterile conditions in a vacuum desiccator until use.
Intestinal Organoid Seeding
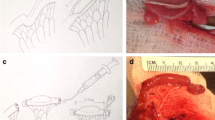
Animals underwent seeding of the intestinal organoids into (a) subcutaneous tissue (n = 44), (b) omentum (n = 6), (c) biopolymer scaffolds wrapped in omentum (n = 15), (d) segments of mid-jejunum whose surface mucosa had been chemically debrided with perfusion of the chelating agent ethylenediamine tetra-acetic acid (EDTA; n = 4), and (e) segments of mid-jejunum whose mucosa had been surgically debrided with a no. 2 surgical curette (n = 10). The intestinal organoids were quantified, resuspended in DMEM-S, and seeded with a pipette at a density of 20,000 organoids per square centimeter in all graft beds (Fig. 1).
Implantation of Organoids into Subcutaneous Tissue
In dog Auto-1, 44 separate 5-mm incisions were made along four rows on the back of the animal, and 20,000 organoids in 500 μl HBSS* were seeded into each subcutaneous pocket with and without matrigel.15 The skin was closed with a 4-0 monocryl suture. The organoids were left in the subcutaneous pockets for 4 weeks. For 22 of the organoid implantations, 5 μl of India ink (Fisher Scientific, Fair Lawn, NJ, USA) were mixed with the organoid suspension to aid in the identification of the implantation site during microscopic examination.
Implantation of Organoids into Omentum
In dog Auto-4, both labeled (n = 3 sites) and unlabeled (n = 3 sites) organoids were seeded into the omentum. The omentum was wrapped and secured around the organoids with 6-0 polypropylene suture.
Implantation of Organoids into Biopolymer Scaffolds
In dog Auto-2, non-labeled organoids were seeded onto the inner lumen of three PGA tubes. In dog Auto-6, the inner lumen of PGA tubes were seeded with either DsRed lentiviral vector transduced organoids (n = 6 tubes) or unlabeled organoids (n = 6 tubes). The seeded PGA tubes were placed on ice for 30 min to allow attachment of the organoids. The tubes were wrapped and secured in omentum with 6-0 silk suture, marked with 6-0 polypropylene sutures, and placed in the abdomen for 4 weeks (Table 1).
Chemical Debridement of Jejunal Mucosa
In dog Auto-3, a 20-cm segment of jejunum 80 cm proximal to the ileocecal valve was isolated with its mesenteric blood supply intact and the continuity of the gastrointestinal tract restored by re-anastomosis. Both ends of the jejunal segment were cannulated. The mucosal epithelium from the isolated jejunum was chemically stripped using a modification of the technique described by us previously.8 In brief, the mesenteric blood vessels were cross-clamped, and the jejunal segment was flushed vigorously with 1 l of 0.9% saline, 4 l of 5.9% PEG solution, 0.9% saline containing 1 mM dithiothreitol (DTT) for 5 min, then 1 mM DTT/27 mM citrate for 15 min and 1 mM DTT/3 mM EDTA for 60 min at a flow rate of 80 cc/min at 39°C. The debrided segment was flushed with 3 l of HBSS* (Mediatech Inc., Herndon, VA, USA) and then seeded with ileal organoids. The proximal and distal ends of the segment were sutured closed with 4-0 polypropylene suture. The segment was placed in the abdomen for 4 weeks.
Surgical Debridement of Jejunal Mucosa (Surgical Mucosectomy)
In dogs Auto-3, 4, and 5 (Table 1), a 20-cm segment of jejunum 80 cm proximal to the ileocecal valve was isolated with its mesenteric blood supply intact and the continuity of the gastrointestinal tract restored by re-anastomosis. The jejunum was opened along its antimesenteric border. Using a no. 2 surgical curette, the mucosa was then scraped off. Bleeding from the graft bed was controlled by compression with gauze soaked in epinephrine solution (10 μg/ml). Then, the intestinal organoids were seeded onto the surface of the denuded intestine, the intestine was sewn closed along its antimesenteric border and its ends with a running 4-0 polypropylene suture, and the segment was placed in the abdomen for 4 weeks.
Once the intestinal organoids were seeded in the respective graft beds, the abdomen was closed in three layers.
Histology
The animals were killed 4 weeks after organoid seeding with pentobarbital overdose (100 mg/kg; Butler Animal Health Supply, Dublin, OH, USA). The seeded subcutaneous tissue, omental tissue, biopolymer implants, and intestinal tissue were harvested and cut transversely into 5-mm sections. Half of the tissue was mounted in OCT compound (Ted Pella Inc., Redding, CA, USA) and used for frozen sections. The other half of the tissue was fixed in 4% phosphate-buffered formalin for 24 h and paraffin-embedded. Frozen sections were cut (5-µm thickness) on a cryostat (Leica, Wetzlar, Germany) and mounted on glass slides. The tissue was mounted with Vectashield hard mount with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA, USA) to stain the nuclei. Every tenth slide was evaluated for a fluorescent signal (DsRed, CFDA, or DiI) that would indicate engraftment of seeded cells. Immediately adjacent sections were stained with hematoxylin and eosin (H&E) and evaluated for presence of neomucosa. Likewise, paraffin-embedded tissue was mounted on glass slides, stained with H&E and evaluated for the presence of neomucosal growth.
Group 2—Allotransplantation
To harvest organoids, fetuses were obtained from an anesthetized pregnant female. A midline laparotomy and a hysterotomy were made under sterile conditions. Each fetus was removed, and its small intestine was harvested and transferred to the laboratory for organoid isolation. Then, the uterus of the mother was removed. The mother animal remained under anesthesia until the stem cell preparation was complete.
Isolation of Fetal Organoids
The intestinal organoids were isolated as described above for experimental group 1 with the following exceptions. PEG and NAC were not utilized, as the fetal intestine did not contain significant mucus. Enzymatic digestion with dispase and collagenase was performed for 25 min at 22°C.
Biopolymer Preparation and Sterilization
The PGA biopolymer tubes were prepared and sterilized as described above for experimental group 1. Due to the availability of biopolymers at the time of the individual experiments, dog Allo-1 had five biopolymer tubes implanted, dogs Allo-2 and Allo-3 each had three biopolymer tubes implanted, and dog Allo-4 had only one biopolymer tube implanted.
Intestinal Organoid Seeding
The intestinal organoids were seeded on the luminal surface of the PGA at a density of 20,000 organoids per square centimeter and implanted into omentum as described for group 1. An unseeded PGA tube was implanted as a negative control. The abdomen was closed in three layers. The biopolymer implants were left in the abdomen for 4 weeks before retrieval.
Immunosuppression
Transplant recipients underwent induction and maintenance immunosuppression to prevent rejection of the intestinal organoids. Each animal received 250 mg of IV solumedrol intraoperatively before implantation of the fetal intestinal organoids. Starting on postoperative day 1, the animals were maintained on oral cyclosporine dosed at 100 mg twice daily (Novartis, New York, NY, USA). Animals received 500 mg methylprednisolone as an intravenous infusion immediately before cell implantation intraoperatively. Postoperative oral prednisone was tapered as follows: 20 mg per os daily × 4 days, 10 mg per os daily × 4 days, then maintenance dose of 5 mg per os daily until the end of the experiment. Systemic cyclosporine levels were checked on postoperative days 5 and 14.
Histology
The animals were killed at 4 weeks after seeding as described for group 1. All tissues were fixed in 4% phosphate-buffered formalin for 24 h and paraffin-embedded and analyzed for presence of neomucosa as described. The total amount of neomucosa per biopolymer tube was calculated by determining the percentage of the available surface area of each tube that was actually covered by neomucosa.
Results
Surgery
All animals survived to the end of the experimental period without complications.
Isolation of Organoids
In the dogs of the pilot group, the ileum used to isolate the ileal organoids contained and released large amounts of mucus during the preparation. This mucus hindered effective enzymatic digestion and release of organoids. A modified isolation protocol using PEG and NAC was devised, which effectively removed the mucus. The harvested organoids microscopically resembled the neonatal rat organoids that we have harvested in previous experiments.8,10,12 The modified harvest protocol was used for all dogs in the autotransplantation group. An average of 1,430,000 ± 530,000 organoids per ileum was obtained. Fetal intestine used in the allotransplantation experiments did not require the use of PEG and NAC in the digestion protocol, as the fetal intestine did not contain any appreciable mucus. The digestion yielded on average 213,000 ± 22,000 organoids per isolation. The organoids obtained from fetal intestine were microscopically indistinguishable from the organoids obtained from the juvenile ileum. Furthermore, when the number of organoids harvested was controlled for by weight of donor tissue, the organoid yield per gram tissue was similar between the autotransplantation and allotransplantation groups. Organoid preparations took 180 to 200 min in all cases, and there was no difference between groups. The recipients were kept under anesthesia while organoid suspensions were prepared.
Labeling of Intestinal Stem Cell Clusters with Fluorescent Markers
In dog Auto-3, an aliquot of organoids were labeled with CFDA before implantation. In dog Auto-4, aliquots of organoids were labeled with either CFDA or DiI before implantation. All organoids labeled with fluorescent vital stains were readily seen under fluorescent microscopy before implantation. Uniform staining of the organoid clusters was achieved with CFDA and DiI (Figs. 2a, b).
Lentiviral Transduction of Organoids with DsRed
In dogs Auto-5 and Auto-6, aliquots of isolated organoids were transduced with DsRed lentivirus before being implanted. Some of these aliquots were directly implanted, while others were transferred into tissue culture to confirm transduction efficiency. After 48 h in tissue culture, transduced organoids expressed the red fluorescent marker DsRed, confirming that the transduction was successful. The cells in the organoids expressed the DsRed marker with high intensity (Figs. 2c, d).
Intestinal Organoid Implantation and Explant Histology
Autotransplantation
In autotransplantation experiments, organoids were implanted into five different graft beds. In the subcutaneous tissue, no evidence of intestinal mucosal growth was observed in any of the 20 engraftment sites. The India ink particles were observed in 22 of the implants marked with the pigment, confirming that the subcutaneous tissue analyzed contained the implantation sites.
In the omentum, both CFDA-labeled, DiI-labeled, DsRed-lentivirus-labeled, and unlabeled control cells were seeded. Groups of DiI- and CFDA-labeled cells were identified in the graft beds by fluorescent microscopy. However, H&E analysis did not reveal any intestinal mucosa (Figs. 3a–c). Examination of the 15 PGA biopolymer tubes that were wrapped in omentum also did not reveal any mucosa. The lumens of the tubes were obliterated, and the scaffold material revealed abundant inflammatory cells and multinucleated giant cells (Fig. 3d).
Organoid implantation into omentum in autotransplantation. Fluorescent (×10, top) and H&E (×4, bottom) images are shown. Groups of CFDA (a) and DiI (b) labeled cells were present in the omentum. However, H&E analysis showed absence of intestinal mucosa (c). PGA biopolymer tubes wrapped in omentum revealed no intestinal mucosa (d).
Chemical debridement of the intestine with 60 min of EDTA perfusion resulted in the dislodgement of approximately 80% of the crypt mucosal cells from the basement membrane. This amount of debridement had been shown to produce an excellent graft bed in rodents.8,12 In dog Auto-3, organoids had been implanted into graft beds that were debrided in this way for 60 min. In sites where labeled organoids had been seeded, some fluorescent cells were observed. However, these fluorescent cells did not co-localize to the DAPI-stained mucosa. This indicated that the regeneration of mucosa in these debrided areas was not the result of organoid engraftment but, rather, restitution from the remaining quantities of native mucosa (Fig. 4a).
Organoid implantation into denuded intestine in autotransplantation. In chemically debrided intestine, groups of CFDA labeled cells were observed; however, the signals did not colocalize to the DAPI-stained enterocytes (a; ×10). In surgically debrided intestine, no DiI (b) or DsRed (c) labeled cells were identified in the graft beds (×2.5).
A total of ten (n = 10) aliquots of organoids were seeded into surgically debrided intestine (Auto-3, Auto-4, and Auto-5). In all sites that were seeded with unlabeled ileal organoids, there was no presence of ileal bile acid transport protein staining by immunostaining with anti-ASBT antibody that cross-reacts with the dog transporter, which might have indicated successful engraftment of ileal organoids.8,16 In sites where labeled organoids had been seeded (DiI, DsRed), no engraftment of fluorescent cells was observed 4 weeks (Figs. 4b, c).
Allotransplantation
Animals had cyclosporine levels drawn on postoperative days 5 and 14. The levels were within therapeutic range (blood concentrations of 400–600 ng/ml), and no dosing adjustments were necessary. After 4 weeks, H&E histology revealed the presence of intestinal mucosa in 11 of 12 biopolymer tubes. Only the one biopolymer tube implanted into Allo-4 failed to generate neomucosa. On gross examination, the biopolymer tubes were completely enveloped with omentum with clearly visible, well-developed blood vessels entering the bioscaffolds. The lumen of 11 of the 12 biopolymer tubes, which proved on histology to have intestinal neomucosa, grossly had large amounts of mucus. On histology, the intestinal mucosa was indistinguishable from native dog intestine in structure and composition, with fully formed crypts and villi (Fig. 5). The enterocytes-and mucus-producing goblet cells were present in the same location and proportions as in native intestine. Furthermore, an extensive submucosal smooth muscle layer was generated, which resembled the native submucosal muscle layer.
Each 1-cm2 long PGA scaffold had a total available surface area of 502.4 mm2 (internal surface area of each tube = 2πrh, where r = 8 mm and h = 10 mm). In the 11 of 12 biopolymer scaffolds in which neomucosa was observed, an average of 303 mm2 per tube of neomucosa was generated. The unseeded control biopolymer tubes demonstrated fibrovascular ingrowth without any evidence of intestinal mucosa.
Discussion
This study represents the first report of the successful generation of intestinal neomucosa using intestinal organoid transplantation in a large animal model. There have been several recent reports of generation of an intestinal mucosal layer in dogs. In these studies, mucosal defects were created in the small bowel and then bridged by decellularized, porcine-derived small intestinal submucosa or an acellular collagen sponge. These scaffolds then became epithelialized from the adjacent native mucosa.17,18 However, this regenerated mucosa was not the result of organoid transplantation; rather, it reflected the remarkable wound healing capacity of the intestinal epithelium when a mucosal injury or defect is created. The ability of intestinal mucosal stem cells to divide and generate more mucosal surface area in response to either injury or to loss of mucosal surface area is well known.19–23 There has been some thought that, perhaps, regeneration of intestinal mucosa on biopolymer scaffolds in this way can generate large amounts of mucosal surface area. However, there are limits to the amount of mucosal regeneration that can take place in response to mucosal injury or loss, and these repair mechanisms cannot replace larger stretches of lost intestine. Loss of intestine leads to mucosal hypertrophy and that results in some functional compensation.24 However, loss of 70–75% of the small intestine overwhelms this intestinal adaptation response and leads to short bowel syndrome.4 With organoid transplantation, the amount of intestinal mucosa that can be generated would, in principle, not be similarly limited. In the future, it may be possible to amplify the intestinal stem cell clusters in vitro to generate vast amounts of intestinal neomucosa.
In this present study, we optimize methods of intestinal organoid isolation from canine juvenile ileum as well as fetal intestine. With both isolation techniques, we were able to obtain large amounts of viable intestinal organoids. When fetal intestinal organoids were allotransplanted onto PGA biopolymer tubes, a significant amount of intestinal neomucosa was generated. This neomucosa resembled native canine intestine in structure and composition. There were the normal proportions of enterocytes and goblet cells, and we observed the development of a well-formed submucosal muscle layer similar to the native canine intestine. In contrast, autotransplantation of juvenile organoids into different graft beds (subcutaneous tissue, omentum, biopolymer scaffolds, and debrided intestine) failed to produce intestinal neomucosa in any of the engraftment sites.
In the autotransplantation experiments, the recipient bed preparation techniques were chosen based on our previous experience with successful organoid transplantation in rodent models. The omentum has been well established to support the growth of intestinal neomucosa after organoid transplantation in rodents.11,13,25–27. Furthermore, we have previously reported successful intestinal resurfacing in rodents.8 In these autotransplantation experiments, a total of 79 seeding experiments were performed with intestinal organoids that were either unlabeled or labeled with different vital stains. In organoids labeled with DiI or CFDA, we observed strong staining of the organoid clusters at the time of seeding. After 4 weeks time, many recipient graft beds still contained fluorescently labeled cells. However, the pattern of fluorescence did not suggest the presence of intestinal mucosa, and subsequent H&E staining confirmed its absence. It is possible that these fluorescently labeled cells represent the persistence of a mesenchymal component of the organoids. A weakness of our studies may be that no further tests were performed to investigate the ratio of labeled mesenchymal and labeled mucosal cells. Thus, we cannot exclude the possibility that rare non-labeled mucosal cells engrafted into a recipient bed but eluded detection. However, in our experiments, the stained cells were mainly used as a guide to help us focus where we would expect to find neomucosa in the recipient segment. The ultimate determination of the presence of neomucosa was made by analysis of H&E-stained slides. In these slides, we specifically looked for mucosal cell formations in the graft beds. In all of the experiments where the organoids were labeled with vital stains, an aliquot of unlabeled organoids was implanted. Since neomucosa was not found in any of these control engraftment sites, it is unlikely that the labeling of organoids itself affected the long-term viability or the implantation of the organoids.
In contrast to the autotransplantation experiments where juvenile organoids were used, the use of fetal intestinal organoids transplanted onto PGA biopolymer tubes generated neomucosa in almost all samples. Only the one PGA tube implanted into Allo-4 failed to generate neomucosa. This is not easily explained; it is unlikely that this lack of engraftment was due to rejection, since there was no histologic evidence for this. In this experimental series, we chose to transplant the organoids onto PGA tubes wrapped in omentum, since this had developed into a gold-standard for testing of neonatal organoids in rodents in our laboratory during the time period the dog studies were conducted. We avoided cell labeling in this case as a possible confounding factor, since any mucosa grown in the confined luminal space of the PGA tube would evidently be derived from the transplanted organoids.
Why autotransplantation of juvenile organoids failed to generate neomucosa whereas allotransplantation of fetal organoids succeeded is not easily explained. However, this result is comparable to previous experience in rodents5,7 (Stelzner, unpublished data). As noted above, generation of small intestinal neomucosa has been reported in different animal species previously when neonatal donors were used. In contrast, successful use of adult organoid donors has never been reported in the literature to our knowledge. It is therefore conceivable that juvenile or adult small intestinal canine organoids do not give rise to a neomucosa, e.g., because they are in some way too differentiated. However, this hypothesis would have to be addressed in future studies.
In both groups, large amounts of organoids were harvested, and equal amounts were seeded onto similar graft beds. It is conceivable that the fetal intestinal organoids are more primitive and more vigorous than the juvenile intestinal organoids. Evidence to support this assumption for enterocytes is sparse, but Guillot et al. has recently shown that fetal mesenchymal stem cells express more pluripotency markers, have longer telomeres, and are more readily expandable and senesce later in culture than their adult counterparts.28,29. The present pilot study has additional limitations since the autotransplantation group is in other aspects not comparable to the allotransplantation group. For example, it is possible that the immunosuppressive medications enhanced organoid implantation or acted as a growth stimulus for the mucosa. Investigation of such drug actions would have exceeded the scope of this study of and would need to be further elucidated.
We have demonstrated in this study that generating intestinal neomucosa with organoid transplantation is feasible in large animals. As with any potential clinical therapy, demonstration of a “proof of principle” is generally accepted as an important milestone before considering human studies. Some obstacles still remain before intestinal organoid transplantation could be used for therapy in human applications such as the treatment of short bowel syndrome or malabsorption syndromes. Currently, no methods exist to successfully harvest and transplant adult intestinal epithelial stem cells, which would appear more widely applicable than transplantation of fetal cells.5 The lack of availability and banking of neonatal or fetal cells from human donors also currently limits the feasibility of this approach for clinical applications. This is not different from several other areas of stem cell transplantation. Furthermore, intestinal organoid transplantation only generates the intestinal mucosal layer. Recently, Nakase et al.30,31 reported that transplantation of smooth muscle cells onto collagen sponge scaffolds results in generation of both an intestinal smooth muscle layer as well as enteroendocrine cells and nerve tissue in the tissue-engineered small intestinal segment. However, generation of a functional, peristaltic neuromuscular unit has still not been reported. Finally, a very large number of transplantable cells would need to be available before attempts at producing bioengineered human intestinal mucosa can be made. In our previous rat model, we were able to produce enough neomucosa using organoid transplantation to cure a clinical malabsorption syndrome.10 This is very encouraging; however, good methods to amplify the stem cell mass to bioengineeer adequately large neomucosal segments in humans are not yet available. A concerted effort to make progress in these areas is necessary for intestinal organoid transplantation to become part of our clinical armamentarium.
Abbreviations
- ASBT:
-
apical sodium bile-acid transporter
- CFDA:
-
carboxyfluorescein diacetate
- DiI:
-
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DMEM:
-
Dulbecco’s modified Eagle medium
- DTT:
-
dithiothreitol
- EDTA:
-
ethylenediamine tetra-acetic acid
- H&E:
-
hematoxylin and eosin
- HBSS*:
-
Hanks’ buffered saline solution
- NAC:
-
N-acetyl cysteine
- OCT:
-
optimal cutting temperature
- PBS:
-
phosphate-buffered saline
- PEG:
-
polyethylene glycol
- PGA:
-
polyglycolic acid
References
Byrne TA, Nompleggi DJ, Wilmore DW. Advances in the management of patients with intestinal failure. Transplant Proc 1996;28(5):2683–2690.
Gazet JC, Kopp J. The surgical significance of the ileocecal junction. Surgery 1964;56:565–573.
Thompson JS. Surgical management of short bowel syndrome. Surgery 1993;113(1):4–7.
Thompson JS. Management of the short bowel syndrome. Gastroenterol Clin North Am 1994;23(2):403–420.
Evans GS, et al. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci 1992;101(Pt 1):219–231.
Tait IS, Penny JI, Campbell FC. Does neomucosa induced by small bowel stem cell transplantation have adequate function? Am J Surg 1995;169(1):120–125. doi:10.1016/S0002-9610(99)80119-6.
Tait IS, et al. Generation of neomucosa in vivo by transplantation of dissociated rat postnatal small intestinal epithelium. Differentiation 1994;56(1–2):91–100. doi:10.1046/j.1432-0436.1994.56120091.x.
Avansino JR, et al. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery 2006;140(3):423–434. doi:10.1016/j.surg.2006.03.012.
Tavakkolizadeh A, et al. Tissue-engineered neomucosa: morphology, enterocyte dynamics, and SGLT1 expression topography. Transplantation 2003;75(2):181–185. doi:10.1097/01.TP.0000044101.03656.9F.
Avansino JR, et al. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg 2005;201(5):710–720. doi:10.1016/j.jamcollsurg.2005.06.270.
Grikscheit TC, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg 2004;240(5):748–754. doi:10.1097/01.sla.0000143246.07277.73.
Avansino JR, et al. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J Surg Res 2006;132(1):74–79. doi:10.1016/j.jss.2005.09.009.
Chen DC, et al. Optical tissue window: a novel model for optimizing engraftment of intestinal stem cell organoids. J Surg Res 2006;134(1):52–60. doi:10.1016/j.jss.2006.03.029.
Horn PA, et al. Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood 2004;103(10):3710–3716. doi:10.1182/blood-2003-07-2414.
Slorach EM, Campbell FC, Dorin JR. A mouse model of intestinal stem cell function and regeneration. J Cell Sci 1999;112(Pt 18):3029–3038.
Stelzner M, Hoagland VD, Woolman JD. Identification of optimal harvest sites of ileal stem cells for treatment of bile Acid malabsorption in a dog model. J Gastrointest Surg 2003;7(4):516–522. doi:10.1016/S1091-255X(03)00027-1.
Chen MK, Badylak SF. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J Surg Res 2001;99(2):352–358. doi:10.1006/jsre.2001.6199.
Hori Y, et al. Tissue engineering of the small intestine by acellular collagen sponge scaffold grafting. Int J Artif Organs 2001;24(1):50–54.
Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol 1999;277(3 Pt 1):G495–G499.
Hudspeth AJ. Establishment of tight junctions between epithelial cells. Proc Natl Acad Sci U S A 1975;72(7):2711–2713. doi:10.1073/pnas.72.7.2711.
Paimela H, Goddard PJ, Silen W. Present views on restitution of gastrointestinal epithelium. Dig Dis Sci 1995;40(11):2495–2496. doi:10.1007/BF02063263.
Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med 2003;31(Suppl8):S532–S537. doi:10.1097/01.CCM.0000081429.89277.AF.
O’Brien DP, et al. Intestinal adaptation: structure, function, and regulation. Semin Pediatr Surg 2001;10(2):56–64. doi:10.1053/spsu.2001.22383.
Helmrath MA, et al. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 1996;183(5):441–449.
Kim SS, et al. Effects of anastomosis of tissue-engineered neointestine to native small bowel. J Surg Res 1999;87(1):6–13. doi:10.1006/jsre.1999.5743.
Kim SS, et al. Regenerative signals for intestinal epithelial organoid units transplanted on biodegradable polymer scaffolds for tissue engineering of small intestine. Transplantation 1999;67(2):227–233. doi:10.1097/00007890-199901270-00007.
Chen DC, et al. Comparison of polyester scaffolds for bioengineered intestinal mucosa. Cells Tissues Organs 2006;184(3-4):154–165. doi:10.1159/000099622.
Guillot PV, et al. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 2007;25(3):646–654. doi:10.1634/stemcells.2006-0208.
Guillot PV, et al. Fetal stem cells: betwixt and between. Semin Reprod Med 2006;24(5):340–347. doi:10.1055/s-2006-952149.
Nakase Y, et al. Endocrine cell and nerve regeneration in autologous in situ tissue-engineered small intestine. J Surg Res 2007;137(1):61–68. doi:10.1016/j.jss.2006.06.019.
Nakase Y, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng 2006;12(2):403–412. doi:10.1089/ten.2006.12.403.
Acknowledgment
Grant support from the Clowes Career Development Award, American College of Surgeons is acknowledged.
Financial disclosures
Authors have no financial arrangements to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Agopian, V.G., Chen, D.C., Avansino, J.R. et al. Intestinal Stem Cell Organoid Transplantation Generates Neomucosa in Dogs. J Gastrointest Surg 13, 971–982 (2009). https://doi.org/10.1007/s11605-009-0806-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0806-x