Abstract
Purpose
Neuromelanin-sensitive MRI (NM-MRI) has proven useful for diagnosing Parkinson’s disease (PD) by showing reduced signals in the substantia nigra (SN) and locus coeruleus (LC), but requires a long scan time. The aim of this study was to assess the image quality and diagnostic performance of NM-MRI with a shortened scan time using a denoising approach with deep learning-based reconstruction (dDLR).
Materials and methods
We enrolled 22 healthy volunteers, 22 non-PD patients and 22 patients with PD who underwent NM-MRI, and performed manual ROI-based analysis. Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) in ten healthy volunteers were compared among images with a number of excitations (NEX) of 1 (NEX1), NEX1 images with dDLR (NEX1 + dDLR) and 5-NEX images (NEX5). Acquisition times for NEX1 and NEX5 were 3 min 12 s and 15 min 58 s, respectively. Diagnostic performances using the contrast ratio (CR) of the SN (CR_SN) and LC (CR_LC) and those by visual assessment for differentiating PD from non-PD were also compared between NEX1 and NEX1 + dDLR.
Results
Image quality analyses revealed that SNRs and CNRs of the SN and LC in NEX1 + dDLR were significantly higher than in NEX1, and comparable to those in NEX5. In diagnostic performance analysis, areas under the receiver operating characteristic curve (AUC) using CR_SN and CR_LC of NEX1 + dDLR were 0.87 and 0.75, respectively, which had no significant difference with those of NEX1. Visual assessment showed improvement of diagnostic performance by applying dDLR.
Conclusion
Image quality for NEX1 + dDLR was comparable to that of NEX5. dDLR has the potential to reduce scan time of NM-MRI without degrading image quality. Both 1-NEX NM-MRI with and without dDLR showed high AUCs for diagnosing PD by CR. The results of visual assessment suggest advantages of dDLR. Further tuning of dDLR would be expected to provide clinical merits in diagnosing PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder involving progressive loss of dopaminergic neurons in the substantia nigra (SN) and noradrenergic neurons in the locus coeruleus (LC), both of which contain pigments called neuromelanin [1, 2]. Neuromelanin is a strong chelator of heavy metals, particularly iron, and plays important roles in protecting against neurotoxicity caused by free iron [3, 4]. Symptoms of PD are thought to appear after 50–60% of dopamine neurons have degenerated, and the presymptomatic phase often spans more than 20 years [5, 6].
Given this background, neuromelanin-sensitive MRI (NM-MRI) methods have been investigated for the early diagnosis of PD [7,8,9,10,11,12,13,14,15,16,17,18,19]. Neuromelanin-iron complexes have T1-shortening effects, and magnetization transfer (MT) effects also contribute to the signal contrast by suppressing signals from background brain parenchyma [7,8,9,10,11,12,13,14,15, 17,18,19,20]. Previous NM-MRI studies have shown decreased signal intensity in the SN of patients with PD [7,8,9,10,11,12,13,14,15,16,17,18,19]. However, NM-MRI requires a relatively long scan time of 7–10 min, because a high resolution with an adequate signal-to noise ratio (SNR) is required due to the small sizes of the SN and LC, and NM-MRI is usually acquired using a number of excitations (NEX) greater than 1 [7,8,9,10, 12,13,14,15, 19]. This long scan time causes difficulty in obtaining sufficient image quality from PD patients with tremor or involuntary movements. A smaller NEX would reduce the scan time, but at the expense of reducing SNR. Although short-scan MR techniques have been tried for NM-MRI (e.g., NM-MRI using a chemical shift selective pulse instead of an MT pulse with scan time of 3 min 20 s and lower image resolution), the diagnostic performance for PD has not been evaluated. [21]. A few reports have described the application of parallel imaging to NM-MRI, and none appear to have used compressed sensing [22, 23].
A denoising approach with deep learning-based reconstruction (dDLR) has been applied for MRI recently [24,25,26,27]. The dDLR is trained using vast amounts of high-quality image data, and makes use of a deep learning neural network to remove image noise and produce clear images in clinical practice. We hypothesized that short-scan time NM-MRI of sufficient image quality would be achievable by applying dDLR to NM-MRI with a fewer NEX. To shorten the scan time as much as possible, we used NEX-1 NM-MRI as source images. To the best of our knowledge, no previous studies have examined the image quality or diagnostic accuracy of NM-MRI with dDLR.
The purposes of this study were thus: (1) to compare image quality between NEX-1 NM-MRI without dDLR, NEX-1 NM-MRI with dDLR, and a reference standard of NEX-5 NM-MRI; and (2) to compare the diagnostic capability of NEX-1 NM-MRI with and without dDLR for differentiating patients with PD from non-PD patients.
Materials and methods
Study population
This prospective study was approved by the institutional ethics committee. Written informed consent was obtained from both healthy volunteers and patients prior to enrolment. For the image quality study, we prospectively enrolled 22 healthy volunteers. We recruited relatively young volunteers, because we needed them to stay still for about 16 min to acquire images from NEX-5 NM-MRI. For the diagnostic performance study, we enrolled 22 patients with PD who agreed to participate in this study, all of whom fulfilled the Movement Disorder Society PD Criteria for the diagnosis of PD [28], and 22 age- and sex-matched non-PD patients. Underlying diseases in the non-PD patients were brain aneurysm (n = 13), old brain infarction or ischemic change (n = 6) and cerebral artery stenosis (n = 3). All the lesions were located outside of the brainstem and no apparent brainstem abnormalities associated with old brain infarction such as Wallerian degeneration were observed. All participants underwent NM-MRI at our hospital between September 2019 and March 2021. No participants were excluded due to insufficient image quality or large brainstem lesions. Figure 1 summarizes the participant inclusion process and image analysis.
Image acquisition
We acquired images from NM-MRI using a 2-dimensional gradient echo (2D-GRE) pulse sequence with MT contrast (MTC) preparation on a 3-T scanner (Vantage Centurian; Canon Medical Systems, Otawara, Japan) with a 32-channel head coil. 1-NEX NM-MRI was acquired from all healthy volunteers, and 5-NEX NM-MRI was acquired from 10 out of 22 volunteers (Fig. 1). For patients with PD, only 1-NEX NM-MRI was performed in this study. Brain MRI for screening had been finished on another day, and no specific abnormalities were found. For non-PD patients, 1-NEX NM-MRI and the other clinical brain scans were performed. Scan parameters were as follows: repetition time /echo time, 460/2.7 ms; 15 slices; slice thickness, 3 mm with no gap; field of view, 230 × 230 mm; matrix, 416 × 416; in-plane resolution, 0.55 × 0.55 mm; flip angle, 40°; bandwidth, 244.1 Hz/pixel; MTC pulses, 300°; off-resonance, 1.2 kHz; and acquisition time, 3 min 12 s for the 1-NEX scan and 15 min 58 s for the 5-NEX scan. No parallel imaging was applied.
Post-imaging procedure
Denoising was applied to 1-NEX images (NEX1) using a commercially available deep learning-based reconstruction algorithm (Advanced intelligent Clear-IQ Engine [AiCE]; Canon Medical Systems) to create NEX1 + dDLR. AiCE is a whole reconstruction pipeline from raw complex data to final image generation. The complex images are processed for denoising in this pipeline which incorporates convolutional neural network. Its details were described in the previous literature [24]. Online Resource 1 displays its architecture. CNN architecture consists of multiple layers: the feature extraction, feature conversion and image generation layers. In the feature extraction layer, the input noisy image is convolved by the 7 × 7 discrete cosine transformation to derive 49 components, which is divided into 48 high-frequency components and a zero-frequency component. A soft-shrinkage activation function is applied to 48 high-frequency components. Next, the 48 high-frequency components undergo repeated 3 × 3 convolution and soft shrinkage in the feature conversion layers. Finally, in the image generation layer, the denoised output image is generated by the 7 × 7 inverse discrete cosine transform convolution of both the output data from the feature conversion layers and the bypassed zero-frequency component. The soft-shrinkage activation function enables adaptive noise removal using a threshold calculated from the noise level and a coefficient. Thus, there are two parameters to be trained: the 3 × 3 convolution kernels in the feature conversion layer and the coefficient of the soft-shrinkage activation function in the feature extraction and feature conversion layers. These parameters have been determined to minimize the differences between the training data and the output denoised image through the training process of AiCE.
Image analysis
All images were analyzed using ImageJ software (National Institutes of Health) as the consensus decisions of 2 board-certified radiologists (S.O. and Y.F. with 9 and 22 years of experience in neuroradiology, respectively). Regions of interest (ROIs) of the SN, decussation of superior cerebellar peduncle (SCP), LC and pons were manually placed on the slice where the SN or LC was most clearly delineated (Online Resource 2). As for the ROIs of the SN, three circles were placed for right and left, respectively, and the signal intensities of the six ROIs were averaged [29]. The SCP and pons were used as background areas for the SN and LC, respectively. The shape and size of ROIs were the same in all images.
-
1.
Image quality
Image quality was assessed quantitatively and qualitatively using images from 10 healthy volunteers and from 22 patients with PD.
For quantitative assessment, we calculated SNR of the SCP (SNR_SCP), SNR of the pons (SNR_pons), contrast-to-noise ratio (CNR) between SN and background SCP (CNR_SN) and CNR between LC and background pons (CNR_LC). We measured signal intensity (SI) in each ROI (SISCP, SIpons, SISN, and SILC). SNR and CNR were defined as follows [30]:
SNR_SCP = mean SISCP / SD of SISCP,
SNR_pons = mean SIpons / SD of SIpons,
CNR_SN = (mean SISN − mean SISCP) / SD of SISCP and
CNR_LC = (mean SILC − mean SIpons) / SD of SIpons, where SD is the standard deviation.
For qualitative assessment of image quality, three neuroradiologists (S.N., S.O. and S.O. with 15, 13 and 10 years of experience in neuroradiology, respectively) visually evaluated overall image quality, artifacts, structural conspicuity and noise of the images at the SN and LC level by consensus using a 5-point Likert scale. The criteria for image assessment on the 5-point Likert scale are presented in Online Resource 3.
-
2.
Diagnostic performance by contrast ratio
To assess diagnostic performance, we calculated contrast ratios (CRs) of the SN and LC using images from 22 non-PD patients, 22 patients with PD and 22 healthy volunteers. CRs were defined as follows:
CR_SN = mean SISN / mean SISCP and
CR_LC = mean SILC / mean SIpons
-
3.
Diagnostic performance by visual assessment
Three neuroradiologists (S.N., S.O. and S.O. with 15, 13 and 10 years of experience in neuroradiology, respectively) visually assessed NEX1 and NEX1 + dDLR images at the level of the SN and LC, respectively, to differentiate PD from non-PD. Raters selected “PD”, “non-PD” and “difficult to diagnose” in accordance with the following criteria. As for SN, raters focused on whether the lateral part of SN is conspicuous or obscure. As for LC, raters focused on whether bilateral high intensities suggesting LC are well defined or not. If it was difficult to determine, “difficult to diagnose” was selected.
Statistical analysis
For quantitative image quality analysis, SNRs and CNRs were compared among NEX1, NEX1 + dDLR and 5-NEX images without dDLR (NEX5) using analysis of variance (ANOVA) with Bonferroni correction for healthy volunteers, and among NEX1 and NEX1 + dDLR using paired t-test for patients with PD. For qualitative analysis, each qualitative index for the three types of images was compared using the Wilcoxon signed-rank test.
For diagnostic performance analysis by contrast ratio, we evaluated difference in CR_SN and CR_LC between age- and sex-matched non-PD and PD groups using Student’s t-test. We then performed receiver operating characteristic curve analyses for differentiating patients with PD from non-PD patients and compared the area under the receiver operating characteristic curve (AUC) between NEX1 and NEX1 + dDLR images using the DeLong test. In addition, differences in CR_SN and CR_LC between healthy and PD groups were assessed and receiver operating characteristic curve analyses were performed.
For diagnostic performance analysis by visual assessment, accuracy was calculated by dividing the number of cases with correct diagnosis by the total number of cases.
MedCalc version 20.009 software (MedCalc Software, Ostend Belgium) was used for statistical analyses, with differences of p < 0.05 considered significant.
Results
The characteristics of participants are listed in Table 1. A total of 66 participants (age range, 26–86 years; mean ± SD age, 56.6 ± 17.0 years; 45 women) were enrolled including 22 healthy volunteers (age range, 26–54 years; mean age, 36.1 ± 7.8 years; 17 women), 22 patients with PD (age range, 58–86 years; mean age, 66.7 ± 9.6 years; 14 women) and age- and sex-matched 22 non-PD patients (age range, 49–82 years; mean age, 67.0 ± 9.1 years; 14 women).
-
1.
Image quality
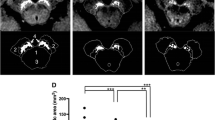
Representative images of the SN and LC from a 30-year-old healthy female volunteer are shown in Fig. 2. NEX1 + dDLR and NEX5 images visualize the SN and LC more clearly than NEX1 images. Results for SNR and CNR of healthy volunteers are presented in Fig. 3 and Table 2. P values are shown in Online Resource 4. SNR and CNR were significantly higher for NEX1 + dDLR than for NEX1 (p < 0.001) at the SN and LC. SNR and CNR from NEX1 + dDLR did not show any significant difference from those of NEX5. For patients with PD, SNR and CNR at the SN and LC were significantly higher for NEX1 + dDLR than for NEX1 (p < 0.001) (Table 2).
Fig. 2 Fig. 3 Box-and-whisker plots and scatter plots for SNR and CNR from NEX1, NEX1 + dDLR and NEX5 images of healthy volunteers. SNR and CNR from NEX1 + dDLR were significantly higher than those from NEX1 and showed no significant difference from those of NEX5. SNR signal-to-noise ratio; CNR contrast-to-noise ratio, NEX number of excitations, dDLR denoising approach with deep learning-based reconstruction
Table 2 Results of SNR and CNR from NEX1, NEX1 + dDLR and NEX5 of healthy volunteers and patients with PD The results of qualitative assessment are shown in Online Resource 5. Scores for overall image quality, structural conspicuity and noise were significantly better for NEX5 among the three types of images (p < 0.01), and those for NEX1 + dDLR were significantly better than those of NEX1 (p < 0.001) for both the SN and LC. No significant differences in scores for artifacts were seen among the three images for both the SN and LC.
-
2.
Diagnostic performance by contrast ratio
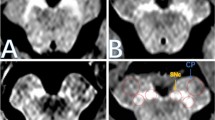
Representative images of the SN and LC from a 73-year-old female non-PD patient and a 54-year-old female patient with PD are shown in Fig. 4. The SN and LC were visualized less clearly for the patient with PD compared to the non-PD patient. CR_SN and CR_LC from patients with PD were significantly decreased compared with those from non-PD patients in both NEX1 (CR_SN, 1.24 ± 0.03 for non-PD and 1.19 ± 0.04 for PD [p < 0.001]; CR_LC, 1.27 ± 0.03 for non-PD and 1.23 ± 0.03 for PD [p < 0.001]) and NEX1 + dDLR (CR_SN, 1.23 ± 0.03 for non-PD and 1.18 ± 0.04 for PD [p < 0.001]; CR_LC, 1.25 ± 0.03 for non-PD and 1.22 ± 0.03 for PD [p = 0.003]) (Fig. 5). The diagnostic performances of NEX1 and NEX1 + dDLR in differentiating PD from non-PD are presented in Table 3. AUCs of NEX1 + dDLR for CR_SN and CR_LC (CR_SN, 0.87 [95% confidence interval (CI), 0.73–0.95]; CR_LC, 0.75 [95%CI, 0.59–0.87]) were not significantly different from those of NEX1 (CR_SN, 0.87 [95%CI, 0.74–0.95]; CR_LC, 0.79 [95%CI, 0.64–0.90]). As for differentiation between healthy volunteers and patients with PD, AUCs of NEX1 + dDLR were 0.92 for CR_SN and 0.82 for CR_LC (Online Resource 6).
Fig. 4 Fig. 5 Box-and-whisker plots and scatter plots of contrast ratios for the substantia nigra and locus coeruleus in patients with PD and non-PD patients from NEX1 and NEX1 + dDLR. Contrast ratios of patients with PD were significantly lower than those of non-PD patients for both images. PD Parkinson’s disease
Table 3 Diagnostic performance of NEX1 and NEX1 + dDLR in differentiating PD from non-PD -
3.
Diagnostic performance by visual assessment
The results of diagnosis by visual assessment are shown in Table 4. Accuracy of each rater for NEX1 and NEX1 + dDLR was 0.45–0.59 and 0.59–0.64 for the SN, while 0.32–0.39 and 0.39–0.41 for the LC, respectively.
Table 4 Diagnostic performance by visual assessment of NEX1 and NEX1 + dDLR for differentiation between healthy volunteers and patients with PD
Discussion
In this study, we applied dDLR to NM-MRI with an NEX of 1, which offers a much shorter scan time than conventional NM-MRI, and examined the resulting image quality and diagnostic performance. Image quality analyses showed approximately 1.5-fold improvement in SNR and CNR by applying dDLR to NEX1 images. The diagnostic performance using CR_SN and CR_LC in NEX1 + dDLR was comparable to that of NEX1. These results may suggest that NEX1 images are sufficient to differentiate between PD and non-PD patients and no apparent benefit for diagnostic performance by dDLR. However, diagnosis by visual assessment showed slight improvement of diagnostic performance by applying dDLR, which suggests the advantages of dDLR in diagnostic performance. Further tuning of dDLR would be expected to provide clinical merits in diagnosing PD. Our study showed a lower diagnostic capability of the LC for PD compared with that of the SN, which was consistent with previous studies [9, 31, 32]. The lower diagnostic performance of the LC than SN and the lower diagnostic performance of NEX1 + dDLR than NEX1 for the LC in our results may be because the LC is so small a structure that limited resolution of MR imaging can make it difficult to quantify the signal intensity accurately. Also, a previous study demonstrated that the optimal flip angle for LC imaging is different from that for SN [33]. Optimization of flip angle to increase contrast of LC may be required for stable measurement of LC and for taking full advantage of dDLR for LC images.
Several articles have already applied deep learning methods to MRI for diagnosing PD, such as for the creation of diagnostic biomarkers for PD [34], automatic segmentation of the SN on NM-MRI [35,36,37], and interpretation of nigrosome 1 on susceptibility map-weighted imaging [38]. However, to the best of our knowledge, no previous studies have applied deep learning-based denoising methods to NM-MRI. Our study demonstrated the utility of using dDLR for NM-MRI to reduce examination times without degrading image quality. Acquisition times for NEX5 and NEX1 are 15 min 58 s and 3 min 12 s, respectively; so, using NEX1 + dDLR instead of NEX5 would achieve a time reduction of around 12 min. This is quite advantageous, particularly for evaluating patients with PD who have tremors or involuntary movements. Furthermore, there is a possibility that denoising by dDLR can be beneficial for diagnosis by visual assessment. Considering that there have not been established criteria for visual diagnosis of PD by NM-MRI, further studies are required in the future.
Several limitations to this study should be considered. First, the number of participants was relatively small. Second, diagnostic performance using NEX5 images was not evaluated. This was because the scan time for NEX5 (15 min 58 s) was too long and uncomfortable for patients with PD, who suffer from tremors or involuntary movements with tolerate. Although we did not compare diagnostic performance between NEX1 + dDLR and NEX5, NEX1 + dDLR showed sufficiently high AUCs (0.87 for CR_SN, 0.75 for CR_LC). Third, age- and sex-matched healthy volunteers were not enrolled, because we recruited relatively young volunteers who could stay still during the scan of about 16 min. Diagnostic performance in this study was therefore evaluated between patients with PD and age- and sex-matched non-PD patients. Fourth, we used 2D NM-MRI in this study and we did not perform voxel-wise analysis as in previous papers [13]. Further investigations should be performed to assess the usefulness of dDLR in the application to 3D NM-MRI. Fifth, various 2D and 3D image sequences including turbo spin echo and GRE have been used for NM-MRI other than the 2D-GRE sequence we used. A future comprehensive study of NM-MRI using these sequences for healthy volunteers and patients with PD is required to determine the most appropriate NM-MRI. Lastly, our study used a vendor-supplied DLR algorithm to assess its clinical feasibility at only one institution. A multicenter study with various MRI scanners is therefore required.
In conclusion, 1-NEX NM-MRI with dDLR provided comparable image quality to 5-NEX NM-MRI, which represented the reference standard in this study. Our study demonstrated the potential of dDLR to reduce scan time of NM-MRI without degrading image quality. The diagnostic performance of 1-NEX NM-MRI using contrast ratio of the SN and that of LC was sufficiently good enough that dDLR did not further improve diagnostic accuracy in this study. However, the results of diagnosis by visual assessment suggest advantages of dDLR. Further tuning of dDLR would be expected not only to reduce scan time of NM-MRI without degrading image quality, but to provide clinical merits in diagnosing PD.
References
Ma SY, Röyttä M, Rinne JO, Collan Y, Rinne UK. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson’s disease using disector counts. J Neurol Sci. 1997;151:83–7.
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–31.
Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E, et al. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J Neural Transm (Vienna). 2006;113:757–67.
Sulzer D, Cassidy C, Horga G, Kang UJ, Fahn S, Casella L, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis. 2018;4:11.
de la Fuente-Fernández R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol. 2011;69:803–10.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301.
Matsuura K, Maeda M, Tabei KI, Umino M, Kajikawa H, Satoh M, et al. A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease. Neurosci Lett. 2016;633:112–7.
Reimão S, Pita Lobo P, Neutel D, Correia Guedes L, Coelho M, Rosa MM, et al. Substantia nigra neuromelanin magnetic resonance imaging in de novo Parkinson’s disease patients. Eur J Neurol. 2015;22:540–6.
Schwarz ST, Xing Y, Tomar P, Bajaj N, Auer DP. In vivo assessment of brainstem depigmentation in Parkinson disease: potential as a severity marker for multicenter studies. Radiology. 2017;283:789–98.
Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. NeuroReport. 2006;17:1215–8.
Trujillo P, Summers PE, Ferrari E, Zucca FA, Sturini M, Mainardi LT, et al. Contrast mechanisms associated with neuromelanin-MRI. Magn Reson Med. 2017;78:1790–800.
Nakane T, Nihashi T, Kawai H, Naganawa S. Visualization of neuromelanin in the Substantia nigra and locus ceruleus at 1.5T using a 3D-gradient echo sequence with magnetization transfer contrast. Magn Reson Med Sci. 2008;7:205–10.
Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci U S A. 2019;116:5108–17.
Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. 2019;142:2558–71.
Chen X, Huddleston DE, Langley J, Ahn S, Barnum CJ, Factor SA, et al. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn Reson Imaging. 2014;32:1301–6.
Martín-Bastida A, Lao-Kaim NP, Roussakis AA, Searle GE, Xing Y, Gunn RN, et al. Relationship between neuromelanin and dopamine terminals within the Parkinson’s nigrostriatal system. Brain. 2019;142:2023–36.
Langley J, Huddleston DE, Chen X, Sedlacik J, Zachariah N, Hu X. A multicontrast approach for comprehensive imaging of substantia nigra. Neuroimage. 2015;112:7–13.
Isaias IU, Trujillo P, Summers P, Marotta G, Mainardi L, Pezzoli G, et al. Neuromelanin Imaging and Dopaminergic Loss in Parkinson’s Disease. Front Aging Neurosci. 2016;8:196.
Ogisu K, Kudo K, Sasaki M, Sakushima K, Yabe I, Sasaki H, et al. 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson’s disease. Neuroradiology. 2013;55:719–24.
Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–44.
Kusama M, Sato N, Kimura Y, Miyagi K. Quick MR neuromelanin imaging using a chemical shift selective pulse. Magn Reson Med Sci. 2021;20:106–11.
Oshima S, Fushimi Y, Okada T, Nakajima S, Yokota Y, Shima A, et al. Neuromelanin-sensitive magnetic resonance imaging using DANTE pulse. Mov Disord. 2021;36:874–82.
Wengler K, He X, Abi-Dargham A, Horga G. Reproducibility assessment of neuromelanin-sensitive magnetic resonance imaging protocols for region-of-interest and voxelwise analyses. Neuroimage. 2020;208: 116457.
Kidoh M, Shinoda K, Kitajima M, Isogawa K, Nambu M, Uetani H, et al. Deep learning based noise reduction for brain MR imaging: tests on phantoms and healthy volunteers. Magn Reson Med Sci. 2020;19:195–206.
Ueda T, Ohno Y, Yamamoto K, Iwase A, Fukuba T, Hanamatsu S, et al. Compressed sensing and deep learning reconstruction for women’s pelvic MRI denoising: Utility for improving image quality and examination time in routine clinical practice. Eur J Radiol. 2021;134: 109430.
Kim M, Kim HS, Kim HJ, Park JE, Park SY, Kim YH, et al. Thin-slice pituitary MRI with deep learning-based reconstruction: diagnostic performance in a postoperative setting. Radiology. 2021;298:114–22.
Wataya T, Nakanishi K, Suzuki Y, Kido S, Tomiyama N. Introduction to deep learning: minimum essence required to launch a research. Jpn J Radiol. 2020;38:907–21.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
Matsuura K, Maeda M, Yata K, Ichiba Y, Yamaguchi T, Kanamaru K, et al. Neuromelanin magnetic resonance imaging in Parkinson’s disease and multiple system atrophy. Eur Neurol. 2013;70:70–7.
Uetani H, Nakaura T, Kitajima M, Yamashita Y, Hamasaki T, Tateishi M, et al. A preliminary study of deep learning-based reconstruction specialized for denoising in high-frequency domain: usefulness in high-resolution three-dimensional magnetic resonance cisternography of the cerebellopontine angle. Neuroradiology. 2021;63:63–71.
Castellanos G, Fernandez-Seara MA, Lorenzo-Betancor O, Ortega-Cubero S, Puigvert M, Uranga J, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord. 2015;30:945–52.
Wang S, Wu T, Cai Y, Yu Y, Chen X, Wang L. Neuromelanin magnetic resonance imaging of substantia nigra and locus coeruleus in Parkinson’s disease with freezing of gait. Front Aging Neurosci. 2023;15:1060935.
Liu Y, Li J, He N, Chen Y, Jin Z, Yan F, et al. Optimizing neuromelanin contrast in the substantia nigra and locus coeruleus using a magnetization transfer contrast prepared 3D gradient recalled echo sequence. Neuroimage. 2020;218: 116935.
Shinde S, Prasad S, Saboo Y, Kaushick R, Saini J, Pal PK, et al. Predictive markers for Parkinson’s disease using deep neural nets on neuromelanin sensitive MRI. Neuroimage Clin. 2019;22: 101748.
Le Berre A, Kamagata K, Otsuka Y, Andica C, Hatano T, Saccenti L, et al. Convolutional neural network-based segmentation can help in assessing the substantia nigra in neuromelanin MRI. Neuroradiology. 2019;61:1387–95.
Krupicka R, Marecek S, Mala C, Lang M, Klempir O, Duspivova T, et al. Automatic substantia nigra segmentation in neuromelanin-sensitive MRI by deep neural network in patients with prodromal and manifest synucleinopathy. Physiol Res. 2019;68:S453–8.
Gaurav R, Pyatigorskaya N, Biondetti E, Valabregue R, Yahia-Cherif L, Mangone G, et al. Deep learning-based neuromelanin MRI changes of isolated REM sleep behavior disorder. Mov Disord. 2022;37:1064–9.
Shin DH, Heo H, Song S, Shin NY, Nam Y, Yoo SW, et al. Automated assessment of the substantia nigra on susceptibility map-weighted imaging using deep convolutional neural networks for diagnosis of Idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2021;85:84–90.
Acknowledgements
The authors are grateful to Nobuyasu Ichinose and Rimika Imai of Canon Medical Systems for their technical support.
Funding
Y.N. Resources: S.O., Y.F., K.K.M., S.N., A.S., S.O., T.H., S.O., H.N., K.F., A.S., M.N., N.S., R.T., T.S. and Y.N. Supervision: Y.F. and Y.N. Institution from which the work originated. Department of Diagnostic Imaging and Nuclear Medicine, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawahara-cho, Sakyo-ku, Kyoto, 606–8507, Japan. This work was supported by an Industry-Academia Collaboration Project, “Research on the development of next-generation medical imaging (No. 151190700001)” provided by Canon Medical Systems Corporation. This work was also supported by JSPS KAKENHI Grant No. 22K07746, 21K15826, 21K20834, and 21K15623.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.O., Y.F., K.K.M., H.N., K.F., M.N. and T.S. Methodology: S.O., Y.F., K.K.M., S.N., A.S., S.O., T.H., S.O., H.N., K.F., M.N., K.U. and T.S. Formal analysis and investigation: S.O. and Y.F. Writing—original draft preparation: S.O. and Y.F. Writing—review and editing: S.O., Y.F., K.K.M., S.N., A.S., S.O., T.H., S.O., H.N., K.F., A.S., M.N., N.S., R.T., T.S. and Y.N.
Corresponding author
Ethics declarations
Conflict of interest
Tsuneo Saga, Kanae Kawai Miyake Hitomi Numamoto and Koji Fujimoto belong to the endowed chair of Industry-Academia Collaboration Project between Kyoto University and Canon Medical Systems Corporation. Masahito Nambu is an employee of Canon Medical Systems Corporation. The remaining authors have no COI.
Ethical statement
This study was approved by the institutional ethics committee. Written informed consent was obtained from both healthy volunteers and patients prior to enrolment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Oshima, S., Fushimi, Y., Miyake, K.K. et al. Denoising approach with deep learning-based reconstruction for neuromelanin-sensitive MRI: image quality and diagnostic performance. Jpn J Radiol 41, 1216–1225 (2023). https://doi.org/10.1007/s11604-023-01452-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-023-01452-9