Abstract
Objective
Endothelial dysfunction is one candidate for triggering neointima formation after arteriovenous grafts (AVGs), but the factors mediating this process are unclear. The purpose of this study was to investigate the role of endoplasmic reticulum stress (ERS)-induced endothelial dysfunction in neointima formation following AVGs in high-fat diet (HFD) mice.
Methods
CCAAT-enhancer-binding protein-homologous protein (CHOP) knockout (KO) mice were created. Mice were fed with HFD to produce HFD model. AVGs model were applied in the groups of WT ND, WT HFD, and CHOP KO HFD. Human umbilical vein endothelial cells (HUVECs) were cultured with oxidized low density lipoprotein (ox-LDL) (40 mg/L) for the indicated time lengths (0, 6, 12, 24 h). ERS inhibitor tauroursodeoxycholic acid (TUDCA) was used to block ERS. Immunohistochemical staining was used to observe the changes of ICAM1. Changes of ERS were detected by real-time RT-PCR. Protein expression levels and ERS activation were detected by Western blotting. Endothellial cell function was determined by endothelial permeability assay and transendothelial migration assay.
Results
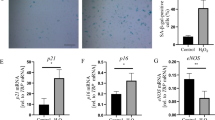
HFD increased neointima formation in AVGs associated with endothelial dysfunction. At the same time, ERS was increased in endothelial cells (ECs) after AVGs in mice consuming the HFD. In vitro, ox-LDL was found to stimulate ERS, increase the permeability of the EC monolayer, and cause endothelial dysfunction. Blocking ERS with TUDCA or CHOP siRNA reversed the EC dysfunction caused by ox-LDL. In vivo, knockout of CHOP (CHOP KO) protected the function of ECs and decreased neointima formation after AVGs in HFD mice.
Conclusion
Inhibiting ERS in ECs could improve the function of AVGs.
Similar content being viewed by others
References
Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation, 2019,139(10):e56–e528
Kulik A, Ruel M, Jneid H, et al. Secondary prevention after coronary artery bypass graft surgery: A scientific statement from the american heart association. Circulation, 2015,131(10):927–964
Xenogiannis I, Zenati M, Bhatt DL, et al. Saphenous vein graft failure: From pathophysiology to prevention and treatment strategies. Circulation, 2021,144(9):728–745
Schlosser A, Pilecki B, Hemstra LE, et al. Mfap4 promotes vascular smooth muscle migration, proliferation and accelerates neointima formation. Arterioscler Thromb Vasc Biol, 2016,36(1):122–133
Luo J, Liang M, Mitch WE, et al. Fsp-1 impairs the function of endothelium leading to failure of arteriovenous grafts in diabetic mice. Endocrinology, 2015,156(6):2200–2210
Wang Y, Liang A, Luo J, et al. Blocking notch in endothelial cells prevents arteriovenous fistula failure despite ckd. J Am Soc Nephrol, 2014,25(4):773–783
Chen S, Ding Y, Tao W, et al. Naringenin inhibits tnf-α induced vsmc proliferation and migration via induction of ho-1. Food Chem Toxicol, 2012,50(9):3025–3031
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol, 2007,8(7):519–529
Nishitoh H. Chop is a multifunctional transcription factor in the er stress response. J Biochem, 2012,151(3):217–219
Okada K, Minamino T, Tsukamoto Y, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation, 2004,110(6):705–712
Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab, 2009,9(1):35–51
Ni L, Zhou C, Duan Q, et al. β-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS one, 2011,6(11):e27294
Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res, 2010,107(7):839–850
Lenna S, Han R, Trojanowska M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB life, 2014,66(8):530–537
Wang XC, Sun WT, Yu CM, et al. Er stress mediates homocysteine-induced endothelial dysfunction: Modulation of ikca and skca channels. Atherosclerosis, 2015,242(1):191–198
Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J, 2016,283(14):2640–2652
Finucane OM, Reynolds CM, Mcgillicuddy FC, et al. Insights into the role of macrophage migration inhibitory factor in obesity and insulin resistance. Proc Nutr Soc, 2012,71(4):622–633
Clarkson-Townsend DA, Douglass AJ, Singh A, et al. Impacts of high fat diet on ocular outcomes in rodent models of visual disease. Exp Eye Res, 2021,204:108440
Cheng J, Wang Y, Liang A, et al. Fsp-1 silencing in bone marrow cells suppresses neointima formation in vein graft. Circ Res, 2012,110(2):230–240
Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol, 2007,27(8):1744–1751
Soejima E, Ohki T, Kurita Y, et al. Protective effect of 3-hydroxybutyrate against endoplasmic reticulum stress-associated vascular endothelial cell damage induced by low glucose exposure. PLoS one, 2018,13(3):e0191147
Eelen G, De Zeeuw P, Simons M, et al. Endothelial cell metabolism in normal and diseased vasculature. Circ Res, 2015,116(7):1231–1244
Rochette L, Meloux A, Rigal E, et al. The role of osteoprotegerin in vascular calcification and bone metabolism: The basis for developing new therapeutics. Calcif Tissue Int, 2019,105(3):239–251
Ding R, Sun X, Yi B, et al. Nur77 attenuates inflammasome activation by inhibiting caspase-1 expression in pulmonary vascular endothelial cells. Am J Respir Cell Mol Biol, 2021,65(3):288–299
Yu FP, Islam D, Sikora J, et al. Chronic lung injury and impaired pulmonary function in a mouse model of acid ceramidase deficiency. Am J Physiol Lung Cell Mol Physiol, 2018,314(3):L406–L420
Ren J, Bi Y, Sowers JR, et al. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol, 2021,18(7):499–521
Sanson M, Augé N, Vindis C, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: Prevention by oxygen-regulated protein 150 expression. Circ Res, 2009,104(3):328–336
Muller C, Salvayre R, Nègre-Salvayre A, et al. Hdls inhibit endoplasmic reticulum stress and autophagic response induced by oxidized ldls. Cell Death Differ, 2011,18(5):817–828
Ubeda M, Wang XZ, Zinszner H, et al. Stress-induced binding of the transcriptional factor chop to a novel DNA control element. Mol Cell Biol, 1996,16(4):1479–1489
Grechowa I, Horke S, Wallrath A, et al. Human neutrophil elastase induces endothelial cell apoptosis by activating the perk-chop branch of the unfolded protein response. FASEB J, 2017,31(9):3868–3881
Hu Y, Lu X, Xu Y, et al. Salubrinal attenuated retinal neovascularization by inhibiting chop-hiflα-vegf pathways. Oncotarget, 2017,8(44):77219–77232
Dai X, Ding Y, Liu Z, et al. Phosphorylation of chop (c/ebp homologous protein) by the amp-activated protein kinase alpha 1 in macrophages promotes chop degradation and reduces injury-induced neointimal disruption in vivo. Circ Res, 2016,119(10):1089–1100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing financial interests or personal relationships that influenced the work reported in this paper.
Additional information
This research was funded by the National Natural Science Foundation of China (No. 81770413) and Hubei Provincial Natural Science Foundation of China (No. 2017CFB669).
Supplementary data
Rights and permissions
About this article
Cite this article
Zhong, Yx., Zhou, Cc., Zheng, Yf. et al. Endoplasmic Reticulum Stress-induced Endothelial Dysfunction Promotes Neointima Formation after Arteriovenous Grafts in Mice on High-fat Diet. CURR MED SCI 43, 115–122 (2023). https://doi.org/10.1007/s11596-022-2663-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2663-8