Summary
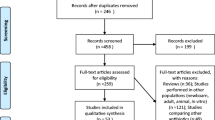
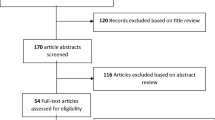
This study aimed to develop a guideline for therapeutic drug monitoring (TDM) of vancomycin. We adopted the new guideline definition from the Institute of Medicine (IOM), adhered closely to the six domains of the Appraisal of Guidelines for Research & Evaluation II (AGREE II), and made recommendations based on systematic reviews. We established a Guideline Steering Group and a Guideline Development Group, formulated 12 questions in the form of Population, Intervention, Comparison, Outcome (PICO) and completed a literature search. As far as we know, we will develop the first evidenced-based guideline for vancomycin TDM under the framework of the Grade of Recommendations Assessment, Development and Evaluation (GRADE).
Similar content being viewed by others
References
Cataldo MA, Tacconelli E, Grilli E, et al. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother, 2012, 67(1): 17–24
Ye ZK, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PloS one, 2013, 8(10): e77169
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm, 2009, 66(1): 82–98
Matsumoto K, Takesue Y, Ohmagari N, et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother, 2013, 19(3): 365–380
Ye ZK, Li C, Zhai SD. Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PloS One, 2014, 9(6): e99044
Institute of Medicine: Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press, 2011
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ, 2008, 336(7650): 924–926
The AGREE Next Steps Consortium. Appraisal of Guidelines for Research and Evaluation, 2009. Available: http://www.agreetrust.org/ (accessed 2 June 2013)
World Health Organization: WHO Handbook for Guideline Development, 2012. Available: http://apps.who.int/iris/bitstream/10665/75146/1/9789241548441_eng.pdf (accessed 2 Nov 2013)
Chen YL, Norris S, Chen H, et al. The development of a global practie guidelines registry platform. 3rd International Society for Evidence-Based Health Care Conference 2014. Taiwan. November 6-9
http://www.guidelines-registry.org/
Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ, 2008, 337: a744
The RIGHT Group: Essential Reporting Items for Practice Guidelines in Healthcare (RIGHT). 2014. cited 2014 http://www.right-statement.org/ (accessed 5 May 2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Zk., Chen, K., Chen, Yl. et al. A protocol for developing a clinical practice guideline for therapeutic drug monitoring of vancomycin. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 469–472 (2016). https://doi.org/10.1007/s11596-016-1610-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1610-y