Summary
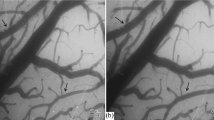
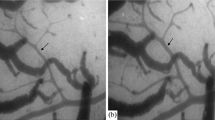
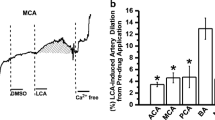
Spontaneous, rhythmical contractions, or vasomotion, can be recorded from cerebral vessels under both normal physiological and pathophysiological conditions. We investigated the cellular mechanisms underlying vasomotion in the cerebral basilar artery (BA) of Wistar rats. Pressure myograph video microscopy was used to study the changes in cerebral artery vessel diameter. The main results of this study were as follows: (1) The diameters of BA and middle cerebral artery (MCA) were 314.5±15.7 μm (n=15) and 233.3±10.1 μm (n=12) at 10 mmHg working pressure (P<0.05), respectively. Pressure-induced vasomotion occurred in BA (22/28, 78.6%), but not in MCA (4/31, 12.9%) from 0 to 70 mmHg working pressure. As is typical for vasomotion, the contractile phase of the response was more rapid than the relaxation phase; (2) The frequency of vasomotion response and the diameter were gradually increased in BA from 0 to 70 mmHg working pressure. The amplitude of the rhythmic contractions was relatively constant once stable conditions were achieved. The frequency of contractions was variable and the highest value was 16.7±4.7 (n=13) per 10 min at 60 mmHg working pressure; (3) The pressure-induced vasomotion of the isolated BA was attenuated by nifedipine, NFA, 18β-GA, TEA or in Ca2+-free medium. Nifedipine, NFA, 18β-GA or Ca2+-free medium not only dampened vasomotion, but also kept BA in relaxation state. In contrasts, TEA kept BA in contraction state. These results suggest that the pressure-induced vasomotion of the isolated BA results from an interaction between Ca2+-activated Cl− channels (CaCCs) currents and KCa currents. We hypothesize that vasomotion of BA depends on the depolarizing of the vascular smooth muscle cells (VSMCs) to activate CaCCs. Depolarization in turn activates voltage-dependent Ca2+ channels, synchronizing contractions of adjacent cells through influx of extracellular calcium and the flow of calcium through gap junctions. Subsequent calcium-induced calcium release from ryanodine-sensitive stores activates KCa channels and hyperpolarizes VSMCs, which provides a negative feedback loop for regenerating the contractile cycle.
Similar content being viewed by others
References
Sakurai T, Terui N. Effects of sympathetically induced vasomotion on tissue-capillary fluid exchange. Am J Physiol Heart Circ Physiol, 2006,291(4):H1761–H1767
Bouskela E, Grampp W. Spontaneous vasomotion in hamster cheek pouch arterioles in varying experimental conditions. Am J Physiol, 1992,262(2 Pt 2):H478–H485
Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol, 1984,246(4 Pt 2):H508–H517
Minamiyama M, Hanai S. Propagation properties of vasomotion at terminal arterioles and precapillaries in the rabbit mesentery. Biorheology, 1991,28(3–4):275–286
Hundley WG, Renaldo GJ, Levasseur JE, et al. Vasomotion in cerebral microcirculation of awake rabbits. Am J Physiol, 1988,254(1 Pt 2):H67–71
Fujii K, Heistad DD, Faraci FM. Ionic mechanisms in spontaneous vasomotion of the rat basilar artery in vivo. J Physiol, 1990,430:389–398
Fujii K, Heistad DD, Faraci FM. Vasomotion of basilar arteries in vivo. Am J Physiol, 1990,258(6 Pt 2):H1829–H1834
Morita-Tsuzuki Y, Bouskela E, Hardebo JE. Vasomotion in the rat cerebral microcirculation recorded by laser-Doppler flowmetry. Acta Physiol Scand, 1992,146(4):431–439
Bertuglia S, Colantuoni A, Intaglietta M. Effects of L-NMMA and indomethacin on arteriolar vasomotion in skeletal muscle microcirculation of conscious and anesthetized hamsters. Microvasc Res, 1994,48(1):68–84
Hill CE, Eade J, Sandow SL. Mechanisms underlying spontaneous rhythmical contractions in irideal arterioles of the rat. J Physiol, 1999,521(Pt 2):507–516
Peng H, Matchkov V, Ivarsen A, et al. Hypothesis for the initiation of vasomotion. Circ Res, 2001,88(8):810–815
Gustafsson H, Nilsson H. Rhythmic contractions of isolated small arteries from rat: role of calcium. Acta Physiol Scand, 1993,149(3):283–291
Rucker M, Strobel O, Vollmar B, et al. Vasomotion in critically perfused muscle protects adjacent tissues from capillary perfusion failure. Am J Physiol Heart Circ Physiol, 2000,279(2):H550–558
Shimamura K, Sekiguchi F, Sunano S. Tension oscillation in arteries and its abnormality in hypertensive animals. Clin Exp Pharmacol Physiol, 1999,26(4):275–284
Griffith TM. Temporal chaos in the microcirculation. Cardiovasc Res, 1996,31(3):342–358
Gratton RJ, Gandley RE, McCarthy JF, et al. Contribution of vasomotion to vascular resistance: a comparison of arteries from virgin and pregnant rats. J Appl Physiol, 1998,85(6):2255–2260
Hudetz AG, Biswal BB, Shen H, et al. Spontaneous fluctuations in cerebral oxygen supply. An introduction. Adv Exp Med Biol, 1998,454:551–559
Haddock RE, Hirst GD, Hill CE. Voltage independence of vasomotion in isolated irideal arterioles of the rat. J Physiol, 2002,540(Pt 1):219–229
von der Weid PY, Beny JL. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J Physiol, 1993,471:13–24
Segal SS, Beny JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol, 1992,263(1 Pt 2):H1–7
Ruehlmann DO, Lee CH, Poburko D, et al. Asynchronous Ca(2+) waves in intact venous smooth muscle. Circ Res, 2000,86(4):E72–79
van Breemen C, Chen Q, Laher I. Superficial buffer barrier function of smooth muscle sarcoplasmic reticulum. Trends Pharmacol Sci, 1995,16(3):98–105
Boedtkjer DM, Matchkov VV, Boedtkjer E, et al. Vasomotion has chloride-dependency in rat mesenteric small arteries. Pflugers Arch, 2008,457(2):389–404
Large WA, Wang Q. Characteristics and physiological role of the Ca(2+)-activated Cl- conductance in smooth muscle. Am J Physiol, 1996,271(2 Pt 1):C435–454
Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol, 2000,74(3–5):175–221
Kitamura K, Yamazaki J. Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn J Pharmacol, 2001,85(4):351–357
Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl− channels—homing in on an elusive channel species. Prog Neurobiol, 2000,60(3):247–289
Griffith TM, Edwards DH. Ca2+ sequestration as a determinant of chaos and mixed-mode dynamics in agonist-induced vasomotion. Am J Physiol, 1997,272(4 Pt 2):H1696–1709
Edwards DH, Griffith TM. Entrained ion transport systems generate the membrane component of chaotic agonist-induced vasomotion. Am J Physiol, 1997, 273(2 Pt 2):H909–920
Gustafsson H, Nilsson H. Rhythmic contractions in isolated small arteries of rat: role of K+ channels and the Na+,K(+)-pump. Acta Physiol Scand, 1994,150(2):161–170
Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol, 2001,281(6):C1769–1775
Jaggar JH, Porter VA, Lederer WJ, et al. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol, 2000,278(2):C235–256
Nelson MT, Cheng H, Rubart M, et al. Relaxation of arterial smooth muscle by calcium sparks. Science, 1995,270(5236):633–637
Faraci FM, Sobey CG. Role of potassium channels in regulation of cerebral vascular tone. J Cereb Blood Flow Metab, 1998,18(10):1047–1063
Jiang XW, Si JQ, Li L, Zhao L, et al. The effects of gap junction on the mesenteric artery contraction activities in normotensive rats and spontaneously hypertensive rats. Chongqing Med (Chinese), 2013,42(12):1365–1367
Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion — what is currently thought? Acta Physiol (Oxf), 2011,202(3):253–269
Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv, 2003,3(2):79–89
Bieger D, Ford CA, Tabrizchi R. Potassium-induced intermittent vasomotion in rat isolated pulmonary artery. J Smooth Muscle Res, 2011,47(1):21–35
Matchkov VV. Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion. Dan Med Bull, 2010,57(10):B4191
Thorn CE, Kyte H, Slaff DW, et al. An association between vasomotion and oxygen extraction. Am J Physiol Heart Circ Physiol, 2011,301(1):H442–449
Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol, 1998,274(6 Pt 1):C1755–1761
Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. Embo J, 1994,13(21):5026–5031
Neylon CB, Lang RJ, Fu Y, et al. Molecular cloning and characterization of the intermediate-conductance Ca(2+)-activated K(+) channel in vascular smooth muscle: relationship between K(Ca) channel diversity and smooth muscle cell function. Circ Res, 1999,85(9):e33–43
Doughty JM, Langton PD. Measurement of chloride flux associated with the myogenic response in rat cerebral arteries. J Physiol, 2001,534(Pt 3):753–761
Lamb FS, Volk KA, Shibata EF. Calcium-activated chloride current in rabbit coronary artery myocytes. Circ Res, 1994,75(4):742–750
Lamb FS, Barna TJ. Chloride ion currents contribute functionally to norepinephrine-induced vascular contraction. Am J Physiol, 1998,275(1 Pt 2):H151–160
Myers JH, Lamb FS, Webb RC. Norepinephrine-induced phasic activity in tail arteries from genetically hypertensive rats. Am J Physiol, 1985,248(3 Pt 2):H419–423
Matchkov VV, Aalkjaer C, Nilsson H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J Gen Physiol, 2004,123(2):121–134
Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol, 2002,545(2):615–627
Wang YZ, Liu ZJ, Li L, et al. Effects of chloride channel blockers on excitatory junction potentials in smooth muscle cells of cochlear spiral modiolar artery in guinea pigs. Sheng Li Xue Bao (Chinese), 2006,58(5):456–462
Li L, Ma KT, Zhao L, et al. Niflumic acid hyperpolarizes the smooth muscle cells by opening BK(Ca) channels through ryanodine-sensitive Ca(2+) release in spiral modiolar artery. Sheng Li Xue Bao (Chinese), 2008,60(6):743–750
Li L, Ma KT, Zhao L, et al. Niflumic acid hyperpolarizes smooth muscle cells via calcium-activated potassium channel in spiral modiolar artery of guinea pigs. Acta Pharmacol Sin, 2008,29(7):789–799
Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol, 1994,112(3):977–984
Criddle DN, de Moura RS, Greenwood IA, et al. Inhibitory action of niflumic acid on noradrenaline- and 5-hydroxytryptamine-induced pressure responses in the isolated mesenteric vascular bed of the rat. Br J Pharmacol, 1997,120(5):813–818
Christ GJ, Moreno AP, Melman A, et al. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am J Physiol, 1992,263(2 Pt 1):C373–383
Christ GJ, Moreno AP, Parker ME, et al. Intercellular communication through gap junctions: a potential role in pharmacomechanical coupling and syncytial tissue contraction in vascular smooth muscle isolated from the human corpus cavernosum. Life Sci, 1991,49(24):PL195–200
Christ GJ, Spray DC, el-Sabban M, et al. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res, 1996,79(4):631–646
Li L, Ma KT, Zhao L, et al. Myoendothelial coupling is unidirectional in guinea pig spiral modiolar arteries. Microvasc Res, 2012,84(2):211–217
Earley S, Resta TC, Walker BR. Disruption of smooth muscle gap junctions attenuates myogenic vasoconstriction of mesenteric resistance arteries. Am J Physiol Heart Circ Physiol, 2004,287(6):H2677–2686
Anderson CM, Lopez F, Zhang HY, et al. Reduced uteroplacental perfusion alters uterine arcuate artery function in the pregnant Sprague-Dawley rat. Biol Reprod, 2005,72(3):762–766
Mauban JR, Lamont C, Balke CW, et al. Adrenergic stimulation of rat resistance arteries affects Ca(2+) sparks, Ca(2+) waves, and Ca(2+) oscillations. Am J Physiol Heart Circ Physiol, 2001,280(5):H2399–2405
Matchkov VV, Rahman A, Peng H, et al. Junctional and nonjunctional effects of heptanol and glycyrrhetinic acid derivates in rat mesenteric small arteries. Br J Pharmacol, 2004,142(6):961–972
Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol, 1997,503(Pt 1):99–110
Tsai ML, Watts SW, Loch-Caruso R, et al. The role of gap junctional communication in contractile oscillations in arteries from normotensive and hypertensive rats. J Hypertens, 1995,13(10):1123–1133
Sell M, Boldt W, Markwardt F. Desynchronising effect of the endothelium on intracellular Ca2+ concentration dynamics in vascular smooth muscle cells of rat mesenteric arteries. Cell Calcium, 2002,32(2):105–120
Tare M, Coleman HA, Parkington HC. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am J Physiol Heart Circ Physiol, 2002,282(1):H335–341
Jackson WF, Mulsch A, Busse R. Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am J Physiol, 1991,260(1 Pt 2):H248–253
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors contributed equally to this work.
This project was supported by grants from National Basic Research Program of China (No. 2012CB52660000), and National Natural Science Foundation of China (No. 81000411, No. 31100829, and No. 31260247).
Rights and permissions
About this article
Cite this article
Li, L., Wang, R., Ma, Kt. et al. Differential effect of calcium-activated potassium and chloride channels on rat basilar artery vasomotion. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 482–490 (2014). https://doi.org/10.1007/s11596-014-1303-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1303-3