Summary
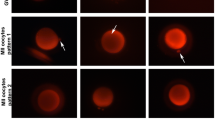
The effects of mouse oocyte vitrification on mitochondrial membrane potential and distribution were explored in this study. The collected mouse oocytes were randomly divided into vitrification and control groups. Ethylene glycol (EG) and dimethylsulphoxide (DMSO) were used as cryoprotectants in the vitrification group. The mitochondrial function and distribution in the oocytes were examined by using the fluorescent probes, JC-1 and Mito Tracker green. The results showed that the ratio of red to green fluorescence in mouse oocytes was significantly decreased after thawing in the vitrification group as compared with the control group (1.28 vs. 1.70, P<0.05). The percentage of polarized distribution of the mitochondria in oocytes was conspicuously reduced in the vitrification group when compared with the control group (31% vs. 63%, P<0.05). It was suggested that vitrification significantly affects the mitochondrial function and distribution in oocytes and reduces the potential of oocyte fertilization and embryo development.
Similar content being viewed by others
References
Chen C. Pregnancy after human oocyte cryopreservation. Lancet, 1986,1(8486):884–886
Dovey S. Oocyte cryopreservation: advances and drawbacks. Minerva Ginecol, 2012,64(6):485–500
Borini A, Bianchi V. Cryopreservation of mature and immature oocytes. Clin Obstet Gynecol, 2010,53(4): 763–774
Lornage J, Salle B. Ovarian and oocyte cryopreservation. Curr Opin Obstet Gynecol, 2007,19(4):390–394
Kuwayama M, Vajta G, Kato O, et al. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online, 2005,11(3):300–308
Antinori M, Licata E, Dani G, et al. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online, 2007,14(1):72–79
Homburg R, van der Veen F, Silber SJ. Oocyte vitrification—women’s emancipation set in stone. Fertil Steril, 2009,91(4 Suppl):1319–1320
Boldt J. Current results with slow freezing and vitrification of the human oocyte. Reprod Biomed Online, 2011,23(3):314–322
Wang Y, Zhu G, Li X, et al. Experimental study on meiotic spindles and chromosomes of mouse mature (M II) stage oocytes under laser scanning confocal microscopy. J Huazhong Univ Sci Technol [Med Sci], 2006,26(3): 353–355
Berthelot-Ricou A, Perrin J, di Giorgio C, et al. Genotoxicity assessment of mouse oocytes by comet assay before vitrification and after warming with three vitrification protocols. Fertil Steril, 2013,100(3):882–888
Ou XH, Li S, Wang ZB, et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod, 2012,27(7):2130–2145
Yongsheng J, Xiaoyun M, Xiaoli W, et al. Antitumor activity of erythromycin on human neuroblastoma cell line (SH-SY5Y). J Huazhong Univ Sci Technol [Med Sci], 2011,31(1):33–38
Reers M, Smiley ST, Mottola-Hartshorn C, et al. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol, 1995,260:406–417
Gosden RG. Fertility preservation: definition, history, and prospect. Semin Reprod Med, 2009,27(6):433–437
Herrero L, Martinez M, Garcia-Velasco JA. Current status of human oocyte and embryo cryopreservation. Curr Opin Obstet Gynecol, 2011,23(4):245–250
Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update, 2012,18(5): 536–554
Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril, 2010,94(6):2088–2095
Nagai S, Mabuchi T, Hirata S, et al. Oocyte mitochondria: strategies to improve embryogenesis. Hum Cell, 2004,17 (4):195–201
Gualtieri R, Iaccarino M, Mollo V, et al. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril, 2009,91(4):1023–1034
Eichenlaub-Ritter U, Wieczorek M, Luke S, et al. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion, 2011,11(5):783–796
Brevini TA, Cillo F, Antonini S, et al. Cytoplasmic remodelling and the acquisition of developmental competence in pig oocytes. Anim Reprod Sci, 2007,98(1–2): 23–38
Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol, 2013,14(3):141–152
Deng WP, Ren ZR. Mitochondrial and oocyte development. Yi Chuan (Chinese), 2007,29(12):1429–1433
Katayama M, Zhong Z, Lai L, et al. Mitochondrial distribution and microtubule organization in fertilized and cloned porcine embryos: implications for developmental potential. Dev Biol, 2006,299(1):206–220
Li Y, Feng HL, Cao YJ, et al. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril, 2006,85(4): 827–832
Yin H, Duffy DM, Gosden RG. Comparative maturation of cynomolgus monkey oocytes in vivo and in vitro. Reprod Biol Endocrinol, 2006,4:14
Torner H, Alm H, Kanitz W, et al. Effect of initial cumulus morphology on meiotic dynamic and status of mitochondria in horse oocytes during IVM. Reprod Domest Anim, 2007,42(2):176–183
Takahashi T, Hammett MF, Cho MS. Multifaceted freezing injury in human polymorphonuclear cells at high subfreezing temperatures. Cryobiology, 1985,22(3):215–236
Lovelock LE. The haemolysis of human red blood cells by freezing and thawing. Biochim Biophys Acta, 1953,10:414–426
Author information
Authors and Affiliations
Corresponding author
Additional information
This project is supported by the Foundation of Hubei Provincial Health Department, China (No. JX5A02).
Rights and permissions
About this article
Cite this article
Lei, T., Guo, N., Tan, Mh. et al. Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 99–102 (2014). https://doi.org/10.1007/s11596-014-1238-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1238-8