Abstract
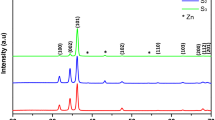
The zinc oxide seed film was coated on conductive glass (FTO) substrate by the Czochralski method, Zinc acetate and hexamethylenetetramine were used as raw materials to prepare growth solution, and then ZnO film was prepared by a low-temperature solution method. The effects of annealing temperature on the morphology, structure, stress and optical properties of ZnO films were studied. The thin films were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-visible absorption spectra (UV — vis), photoluminescence (PL) and X-ray photoelectron spectroscopy (XPS). The results show that the films are ZnO nanorods. With the increase of annealing temperature, the diameter of the rod increases, and the nanorods tend to be oriented. The band gap of the sample obtained from the light absorption spectra first increases and then decreases with the increase of annealing temperature. When the annealing temperature is 350 °C, the crystallinity of zinc oxide film is the highest, the band gap is close to the theoretical value of pure ZnO.
Similar content being viewed by others
References
Ma Z H, Ren F Z, Ming X L, et al. Cu-Doped ZnO Electronic Structure and Optical Properties Studied by First-Principles Calculations and Experiments[J]. Materials, 2019, 12(1): 196
Deng Y F, Ma Z H, Ren F Z, et al. Improved Photoelectric Performance of DSSCs Based on TiO2 Nanorod Array/Ni-doped TiO2 Compact Layer Composites Film[J]. J. Solid State Electrochem., 2019, 23: 3 031–3 041
Deng Y F, Ma Z H, Ren F Z, et al. Enhanced Morphology and Photoelectric Properties of One-dimensional TiO2 Nanorod Array Films[J]. Chem. Phys. Lett., 2019, 724: 42–49
Wu K X, Zha W S, Chen X. Photocatalytic Activity of TiO2 Coatings Fabricated on Al2O3 by Mechanical Coating Technique[J]. J. Wuhan University of Technology-Mater. Sci. Ed., 2021, 36(1): 1–5
Zheng J H, Song J L, Jiang Q, et al. Optical Properties of Cu-doped ZnO Nanoparticles Experimental and First-Principles Theory Research[J]. J Mater Sci. Mater Electron., 2012, 23(8): 1 521–1 524
Liu L Q, Cao G X, Hong K Q. Seed Free Growth of Aligned ZnO Nanowire Arrays on AZO Substrate[J]. J. Wuhan University of Technology-Mater. Sci. Ed., 2018, 33(6): 1 372–1 375
Ran F Y, Tanemura M, Hayashi Y, et al. Effect of Substrate Temperature on the Room-Temperature Ferromagnetism of Cu-doped ZnO Film[J]. J. Cryst. Growth, 2009, 31: 4 270–4 274
Lue J G, J. Dai J, Zhu J B, et al. Effect of Na Concentrations on Microstructure and Optical Properties of ZnO Films[J]. J. Wuhan University of Technology-Mater. Sci. Ed., 2011, 26(1): 23–27
Yu L P. Development in p-type Doping of ZnO[J]. J. Wuhan University of Technology-Mater. Sci. Ed., 2012, 27(6): 1 184–1 187
Zheng H R, Jiang Y R, Yang S Y, et al. ZnO Nanorods Array as Light Absorption Antenna for High-gain UV Photodetectors[J]. J. Alloy. Compd., 2020, 812: 152 158
Bhogaita M, Devaprakasam D. Hybrid Photoanode of TiO2-ZnO Synthesized by Co-precipitation Route for Dye-sensitized Solar Cell using Phyllanthus Reticulatas Pigment Sensitizer[J]. Sol Energy., 2021, 214: 517–530
Shanmuganathan G, Banu I B S, Krishnan S, et al. Influence of K-doping on the Optical Properties of ZnO Thin Films Grown by Chemical Bath Deposition Method[J]. J. Alloy. Compd., 2013, 562: 187–193
Jang S, Son P, Kim J. K Doping Effect on Structural and Optical Properties of ZnO Nanorods Grown on Semipolar (1122) GaN Films Using a Hydrothermal Growth Method[J]. Opt. Mater. Express., 2015, 5: 1 621
Keshtkar J, Vargas G, Roberto J, et al. Preparation of Rod-like Aluminum Doped Zinc Oxide Powders by Sol-gel Technique Using Metal Chlorides and Acetylacetone Precursors[J]. J. Wuhan University of Technology-Mater. Sci. Ed., 2018, 33(6): 1 293–1 297
Yang J J, Fang Q Q, Wang B M, et al. ZnO Based Luminous and Diluted Magnetic Semiconductors Prepared by PVA Methods[J]. Chin. J. Lumin., 2006, 27(6): 939–943
Bharathi V, Sivakumar M, Udayabhaskar R, et al. Structural, Enhanced Local Vibrational and Fluorescence Properties in K-doped ZnO Nanostructures[J]. Appl. Phys. A, 2014, 116(1): 395–401
Ma Z H, Ren F Z, Deng Y F, et al. Experimental and Theoretical Studies of KxZn1−xO[J]. Ceram. Int., 2020, 46: 763–767
Qiu D J, Wu H Z, Feng A M, et al. Annealing Effects on the Microstructure and Photoluminescence Properties of Ni doped ZnO Films[J]. Appl. Surf. Sci., 2004, 222: 263–268
Kadam A N, Kim T G, Shin D S, et al. Morphological Evolution of Cu Doped ZnO for Enhancement of Photocatalytic Activity[J]. J. Alloy. Compd., 2017, 710: 102–113
Sonkera R K, Sikarwarb S, Sabhajeetb S R, et al. Spherical Growth of Nanostructures ZnO Based Optical Sensing and Photovoltaic Application[J]. Opt. Mater., 2018, 83: 342–347
Guo L J, Ye Z Z, Huang J G. Influeruce of Pudeposition Annealing on Crytallinity of Zinc Oxide Flims[J]. Chin. J. Semicond., 2003, 24(7): 370–377
Kim S K, Kim S, Lee C H, et al. The Structural and Optical Behaviors of K-ZnO/Al2O3 (0001) Films[J]. Appl. Phys. Lett., 2004, 85(3): 419–421
Ma Z H, Ren F Z, Deng Y F, et al. Structural, Electrochemical and Optical Properties of Ni Doped ZnO: Experimental and Theoretical Investigation[J]. Optik, 2020, 219: 165 204
Trunk M, Venkatachalapathy V, Galeckas A, et al. Deep Level Related Photoluminescence in ZnMgO[J]. Appl. Phys. Lett., 2010, 97: 211 901
Sun S, Wu P, Xing P. d0 Ferromagnetism in Undoped n and p-type In2O3 Firms[J]. Appl. Phys. Lett., 2012, 101: 132 417
Deng Y F, Ma Z H, Ren F Z. Enhanced Photoelectronchemical Performance of TiO2 Nanorod Array Films Based on TiO2 Compact Layers Synthesized by a Two-step Method[J]. Rsc Adv., 2019, 9: 21 777–21 785
Park C H, Zhang S B, Wei S H. Origin of p-type Doping Difficulty in ZnO: the Impurity Perspective[J]. Phys. Rev. B, 2002, 66: 073 202
Dixit H, Saniz R, Lamoen D, et al. The Quasiparticle Band Structure of Zincblende and Rocksalt ZnO[J]. J. Phys. Condens. Mat., 2010, 22: 125 505
Funding
Funded by Henan International Science and Technology Cooperation Program (No.152102410035), and Ph D Research Startup Foundation of Henan University of Science and Technology(No.13480107)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Z., Ren, F. & Yang, Z. Structure and Optical Properties of ZnO Thin Films Prepared by the Czochralski Method. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 37, 823–828 (2022). https://doi.org/10.1007/s11595-022-2602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-022-2602-3