Abstract
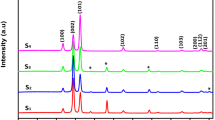
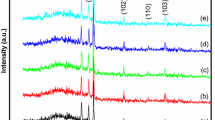
In recent years, metal oxide semiconductors have been one of the most remarkable subjects in science and technology. Present research investigates the growth and characterization of Zinc Oxide (ZnO) nanorods (NRs) as one of the metal oxide semiconductors with a broad range of applications. In this work, ZnO NRs preparation includes two steps. In the first step, Zn thin layers were deposited by thermal evaporation method on soda lime glass (SLG) substrate under an electric field of 134 kV/m. The second step contains the oxidation process of the Zn layers under electric fields of 0, 48 and 84 kV/m in air. Then, samples were characterized by X-ray Diffraction (XRD), Energy Dispersive X-ray Spectroscopy (EDX), Field Emission Scanning Electron Microscopy (FESEM), Raman and UV–Visible Spectroscopy. XRD study showed that all samples were crystallized in a hexagonal wurtzite crystal structure. EDX analysis showed that Zn and O atomic ratio, with a good approximation, are close to the ratio expected for ZnO (1:1). FESEM images illustrated that by applying the electric field, diameter of ZnO NRs decreased. Moreover, Raman spectra indicated a shift towards higher wavenumbers due to decreasing the crystallite size and vice versa. In addition, the results obtained from optical measurement demonstrated a reversed behavior of the samples' band gap energy with crystallite size.







Similar content being viewed by others
References
L. Nánai, M. Füle. Stimulated oxidation of metals (laser, electric field, etc.): comparative studies. In: AIP Conference Proceedings. American Institute of Physics (2014).
N. Tiwale et al., Optimization of transistor characteristics and charge transport in solution processed ZnO thin films grown from zinc neodecanoate. Electron. Mater. Lett. 15(6), 702–711 (2019)
N. Tripathy, D.-H. Kim, Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 5(1), 27 (2018)
R. García-Gutiérrez et al. Luminescence and structure of ZnO grown by physical vapor deposition. Adv. Mater. Sci. Eng. 2012 (2012).
E.W. Petersen et al., Growth of ZnO nanowires catalyzed by size-dependent melting of Au nanoparticles. Nanotechnology 20(40), 405603 (2009)
G. Jimenez-Cadena et al., Synthesis of different ZnO nanostructures by modified PVD process and potential use for dye-sensitized solar cells. Mater. Chem. Phys. 124(1), 694–698 (2010)
F. Xue et al., Influence of external electric field on piezotronic effect in ZnO nanowires. Nano Res. 8(7), 2390–2399 (2015)
F. Xiu et al., p-type ZnO films with solid-source phosphorus doping by molecular-beam epitaxy. Appl. Phys. Lett. 88(5), 052106 (2006)
J.-W. Hoon et al., Direct current magnetron sputter-deposited ZnO thin films. Appl. Surf. Sci. 257(7), 2508–2515 (2011)
Y. Yang et al., ZnO nanoparticles prepared by thermal decomposition of β-cyclodextrin coated zinc acetate. Chem. Phys. Lett. 373(1–2), 22–27 (2003)
A. Ramdas Bari, P. Anil Bari, R.H. Bari, Studies on sol-gel dip-coated nanostructured ZnO thin films. J. Nanostruct. 9(2), 326–330 (2019).
F. Konan et al., Optical-Structural Characteristics of i-ZnO Thin Films Deposited by Chemical Route. (2019).
Ö.A. Yıldırım, C. Durucan, Synthesis of zinc oxide nanoparticles elaborated by microemulsion method. J. Alloy. Compd. 506(2), 944–949 (2010)
A. Rezaee et al., Optimization of combined photocatalytic involving immobilized ZnO nanoparticles and electrochemical processes for ammoniacal nitrogen removal from aqueous solutions. Mater. Environ. Sci 3(5), 955–966 (2012)
N. Winkler et al., Mg-doped ZnO films prepared by chemical bath deposition. J. Mater. Sci. 53(7), 5159–5171 (2018)
S. Benramache et al., Transition width effect on optical characterizations of ZnO thin films deposited by spray ultrasonic. Inorgan. Nano-Met. Chem. 49(6), 177–181 (2019)
P. Listewnik et al., Preparation and characterization of microsphere ZnO ALD coating dedicated for the fiber-optic refractive index sensor. Nanomaterials 9(2), 306 (2019)
F.U. Hamelmann, Thin film zinc oxide deposited by CVD and PVD. In: Journal of Physics: Conference Series. IOP Publishing (2016).
O. Tigli, J. Juhala, ZnO nanowire growth by physical vapor deposition. In 2011 11th IEEE International Conference on Nanotechnology. IEEE, New York (2011).
D. Pradhan, K. Leung, Vertical growth of two-dimensional zinc oxide nanostructures on ITO-coated glass: effects of deposition temperature and deposition time. J. Phys. Chem. C 112(5), 1357–1364 (2008)
M. Jouya, F. Taromian, S. Siami, Rapid growth of zinc oxide nanobars in presence of electric field by physical vapor deposition. J. Theor. Appl. Phys. 11(4), 291–299 (2017)
M. Afshari, M. Ghazi, M. Izadifard, Structural and magnetic properties of Fe/Cu/Fe trilayers. Afr. Rev. Phys. 10 (2015).
M.H. Amerioun et al., Fabrication of CuInS2/CNTs absorber layers by sol–gel method. Mater. Sci. Semicond. Process. 38, 149–156 (2015)
M. DasariAyodhya et al., Synthesis, Characterization of ZnS Nanoparticles by Coprecipitation Method Using Various Capping Agents-Photocatalytic Activity and Kinetic Study.
S. Terohid et al., Effect of growth time on structural, morphological and electrical properties of tungsten oxide nanowire. Appl. Phys. A 124(8), 567 (2018)
S.N. Alam et al., SEM, EDX & XRD of zinc oxide nanostructures synthesized by zinc oxidation. Microsc. Anal. 26(4), 11–14 (2012)
A.F. Abdulrahman et al., Different substrates effects on the topography and the structure of the ZnO nanorods grown by chemical bath deposition method. Digest J. Nanomater. Biostruct. 11(3), 1007–1016 (2016)
N. Neykova et al., Study of ZnO nanorods grown under UV irradiation. Appl. Surf. Sci. 472, 105–111 (2019)
I. Musa, N. Qamhieh, S.T. Mahmoud, Synthesis and length dependent photoluminescence property of zinc oxide nanorods. Results Phys. 7, 3552–3556 (2017)
R. Zhang et al., Photoluminescence and Raman scattering of ZnO nanorods. Solid State Sci. 11(4), 865–869 (2009)
G.-C. Yi, C. Wang, W.I. Park, ZnO nanorods: synthesis, characterization and applications. Semicond. Sci. Technol. 20(4), S22 (2005)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jouya, M., Taromian, F. & Afshari Abolkarlou, M. Effect of an applied electric field during the oxidation process of zinc thin films on growth and properties of ZnO nanorods. Appl. Phys. A 126, 697 (2020). https://doi.org/10.1007/s00339-020-03884-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-03884-w